GOTHENBERG, Sweden — Scientists have developed a drug capable of annihilating the most lethal brain tumors. This drug, dubbed Z4P, successfully breaches the blood-brain barrier – a protective layer that shields neurons from foreign threats – and effectively eradicates cancerous cells.
In a series of experiments, this small molecule was found to eliminate all tumor cells while sparing healthy tissue. These results were described as “very promising,” as mice subjected to this treatment experienced no relapse even after six months. Z4P works synergistically with chemotherapy. In comparison, tumors rapidly recurred and spread in subjects receiving only chemotherapy. This underlines the urgent need for improved therapies for glioblastomas, which are frequently incurable.
The international team behind Z4P believes it could enter clinical practice within five years, offering a significant breakthrough with potential implications for other aggressive cancers.
“These are the first clear results with brain tumors that can lead to a treatment which completely avoids surgery and radiation. We have also begun studying the use of our substance on other aggressive tumor forms like pancreatic cancer, triple-negative breast cancer and certain lever cancers,” says Professor Leif Eriksson from the University of Gothenburg in a media release.
Glioblastomas are notoriously lethal
According to the National Brain Tumor Society, more than 10,000 individuals in the United States will succumb to glioblastoma each year. Fewer than seven percent of patients with this form of cancer survive more than five years after diagnosis. Concerningly, survival rates and mortality statistics have been virtually unchanged for decades.
Z4P operates by blocking a mechanism that stimulates protein production in the tumor, which induces cell death due to stress. Cancer cells, particularly those forming aggressive tumors, hijack healthy cells in a desperate bid for survival.
“We have now succeeded in stopping this hijacking by inserting a specially developed molecule in the cells that inhibits one of these hijacked adaptive mechanisms in the cancer cells. This causes the cancer to self-destruct,” says Leif Eriksson, a professor of physical chemistry.
Scroll down to see why it takes so long for cancer drugs to reach patients
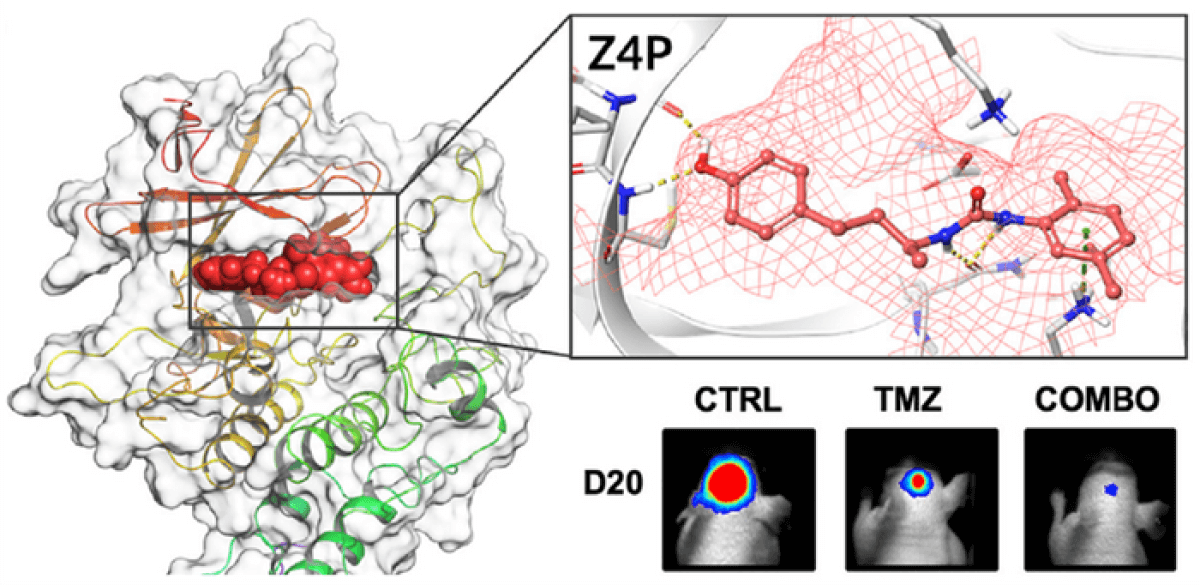
Advanced computer simulations were used to refine a version of Z4P that could penetrate the blood-brain barrier, which typically prevents most pharmaceuticals from being absorbed.
“Today, cancer treatment consists of surgery, radiation and chemotherapy. Unfortunately, all cancer cells are not killed and the tumor returns. Once the cancer relapse, the tumor cells have often spread and developed resistance,” Eriksson adds.
This technique, as described in the journal iScience, does not apply to other forms of brain cancer due to their distinct developmental pathways. Current treatments for brain tumors are often accompanied by severe side effects, none of which were identified with Z4P.
Animals treated with Z4P maintained their weight, showed no discernible behavioral changes, and exhibited no signs of liver damage. Extensive lab tests on cells indicated that the substance is non-toxic, even at very high doses.
Prof Eriksson and his team are now optimizing the treatment procedure and planning further animal studies, yet they remain confident that Z4P should be swiftly integrated into clinical treatment.
“It largely depends on whether funding comes in that allows taking the different steps as smoothly as possible. If I’m optimistic, perhaps it might take five years. That’s a short timeframe, but at the same time glioblastomas are nearly 100 per cent fatal, so any improvement in medical care is major progress,” Eriksson concludes.
What steps do cancer drugs go through to reach patients?
Developing a new drug, including those used to treat cancer, is a complex and rigorous process that can take up to several years. This process is carefully regulated to ensure the safety and effectiveness of the drug. The process includes the following stages:
Preclinical Testing: This is the initial stage of drug development. It involves laboratory experiments to understand the drug’s effect on cancer cells. This phase includes both in vitro (in the lab, often in petri dishes) and in vivo (in animal models) testing. If the drug shows promising results, it moves to the next phase.
Investigational New Drug Application (IND): In this step, the drug’s developers compile the results from the preclinical testing and submit an IND application to a regulatory agency such as the U.S. Food and Drug Administration (FDA). This application should contain data from the preclinical testing, proposed clinical trial protocols, data about the drug’s manufacturing, and plans for patient safety monitoring. If the application is approved, the drug can move to clinical trials.
Clinical Trials: Clinical trials involve testing the drug in humans and are generally conducted in three phases:
- Phase I: This is the first stage of testing in humans. The primary goal is to evaluate the drug’s safety, determine a safe dosage range, and identify side effects. This phase often includes a small number of healthy volunteers or patients.
- Phase II: If Phase I is successful, the drug moves to Phase II testing, where the focus is on evaluating the drug’s effectiveness and further assessing its safety. This phase involves more participants who have the condition that the drug is intended to treat.
- Phase III: This phase involves randomized and blind testing in several hundred to several thousand patients. The aim is to confirm the drug’s effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug to be used safely.
New Drug Application (NDA): If the results from the clinical trials are positive, the drug’s developers can submit an NDA to the FDA (or a similar application to other national regulatory bodies). This application includes full reports of the drug’s testing results, proposed labeling, and information about how it’s manufactured, processed, and packaged.
FDA Review: The FDA then reviews the NDA. If the FDA approves the application, the drug becomes available for physicians to prescribe to patients.
Post-Marketing Surveillance (Phase IV Trials): After a drug is approved, it’s continually monitored for safety in what are known as Phase IV trials. If new side effects or problems are detected, the FDA can choose to remove the drug from the market or change its usage guidelines.
It’s important to note that many drugs never make it through this entire process. It’s estimated that for every 5,000 to 10,000 compounds that enter preclinical testing, only one is approved for patient use.
South West News Service writer Mark Waghorn contributed to this report.

“tumors rapidly recurred and spread in subjects receiving only chemotherapy”
Google “chemotherapy metastases”.
It’s commonly known among oncologists that chemo causes cancer to spread throughout the body. And chemo also creates new cancer stem cells, which can’t be killed by chemotherapy.
Google “Common Cancer Treatments May Create Dangerous Cancer Stem Cells”