SYDNEY, Australia — Regardless of age, back pain is a common problem for many. Spinal cord stimulation is becoming a more common treatment to help relieve the pain, but researchers in Australia say it isn’t that simple. Their new research finds that the technology not only doesn’t relieve pain in the long-term, but it might even cause harm.
Spinal cord stimulation is supposed to work by implanting a device, usually consisting of tiny wires, that sends electrical pulses to the spinal cord. The pulses are meant to interrupt painful nerve signals before they reach the brain, serving as a viable option for treatment with very little disruption to day-to-day life.
In a thorough review of 13 published clinical trials including almost 700 participants, researchers from the University of Sydney compared spinal cord stimulation treatment with placebo or no treatment for back pain. Their results reveal that spinal cord stimulation is actually no better than a placebo, and offers little, if any, benefit for people with low back pain.
Further, the team found that adverse side-effects to the surgery were largely poorly documented, and the associated risks are inconclusive. Potential harms from this form of treatment could include nerve damage, infection, and wire movement — all of which may require more invasive surgical intervention to fix.
“Spinal cord stimulation is invasive and has a great financial cost to people who choose surgery as a last resort to alleviate their pain. Our review found that the long-term benefits and harms are essentially unknown,” says lead researcher Dr. Adrian Traeger from Sydney Musculoskeletal Health, an initiative of the University of Sydney, Sydney Local Health District, and Northern Sydney Local Health District, in a media release.
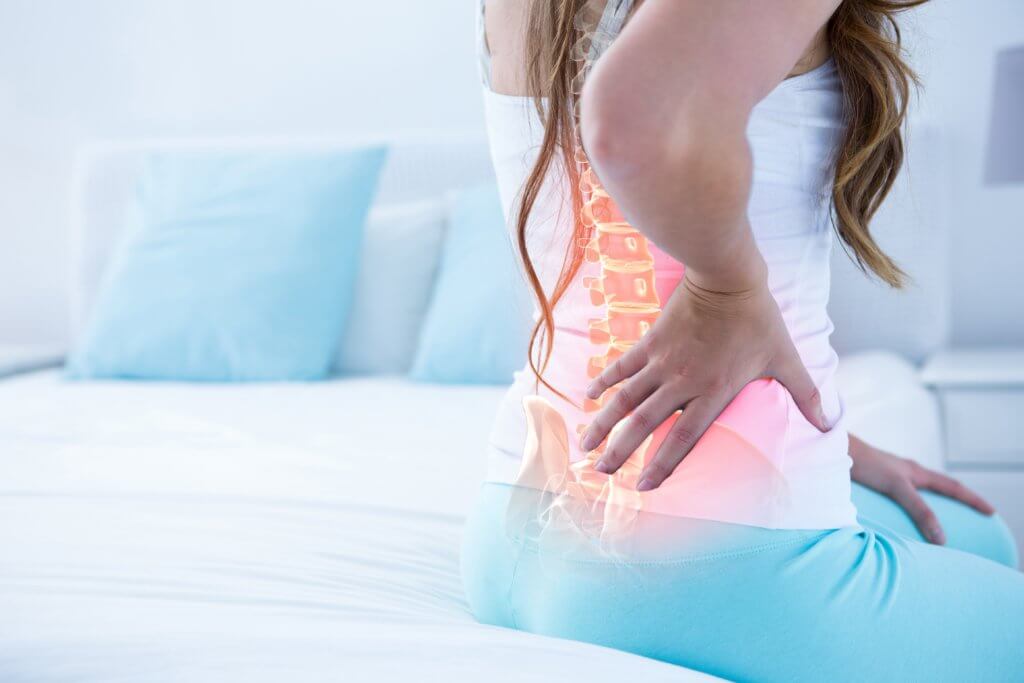
‘Low back pain is one of the leading causes of disability worldwide’
The team notes that there were several data gaps discovered in their review. For one, none of the studies looked at long-term (one year or longer) impacts of spinal cord stimulation. In fact, the longest trial was only six months. Most of the trials looked at immediate effects of its use, which was usually under a month’s time.
“Low back pain is one of the leading causes of disability worldwide. Our findings further emphasize the urgent need to review funding arrangements for chronic pain care to help patients in their search for relief. There are evidence-based physical and psychological therapies for back pain; ensuring access to these is essential,” Dr. Traeger explains.
The review team has recommended ways to move forward, such as having future trials last a minimum of 12 months. They also encourage researchers to provide clear documentation of adverse effects and to compare other treatment options in order to strengthen research quality on the topic, as well as care for these patients.
The findings appear in the journal Cochrane Database of Systematic Reviews.


Exploring the nuances of back pain treatments like spinal cord stimulation underscores the importance of comprehensive and patient-centric approaches. While the findings on spinal cord stimulation are enlightening, it’s essential to consider other avenues for holistic pain management. My recent article, “Comparing Chiropractic and Physiotherapy Protocols with a Focus on Patient Education for Empowered Rehabilitation” , dives deep into the protocols of both chiropractic and physiotherapy. It emphasizes the empowerment of patients through education for more informed decisions in rehabilitation.