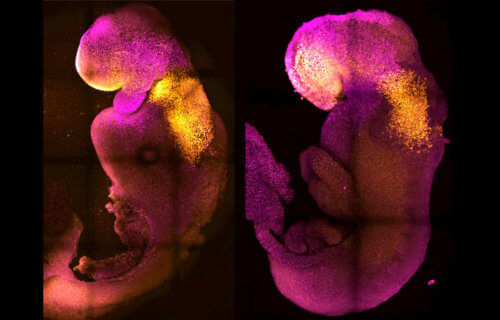
PASADENA, Calif. — A “synthetic” embryo with a brain and beating heart, created by an international team of scientists, could be the first step in understanding why some pregnancies fail.
The embryo features the foundations of all the body’s organs, including neural and gut tubes that protect the developing spine and intestines. The world-first creation could also solve the organ donor shortage crisis. The team from University of Cambridge and Caltech say the embryos may guide the repair and development of synthetic human organs.
Researchers used a combination of stem cells from mice without fertilized eggs or sperm.
“Our mouse embryo model not only develops a brain, but also a beating heart, all the components that go on to make up the body,” says lead author Professor Magdalena Zernicka-Goetz in a media release.
“It’s just unbelievable that we’ve gotten this far. This has been the dream of our community for years, and the major focus of our work for a decade, and finally we’ve done it.”
How does an embryo evolve into a fetus?
The embryo started to grow muscles, a gut, and nervous system, shedding new light on how tissues form and the causes of genetic diseases. Successful pregnancies need a “dialogue” between an embryo and mother. In the first week after fertilization, three types of stem cells develop.
One will eventually become the tissues of the body. The others support the embryo’s development. They become the placenta, which connects the fetus to the mother and provides oxygen and nutrients, and the yolk sac, where the embryo gets its nutrients and grows.
Each group has to send mechanical and chemical signals to each other, telling the embryo how to develop properly. Prof. Zernicka-Goetz notes that so many pregnancies fail during this stage, before most women realize they’re pregnant.
“This early period is the foundation for everything else that follows in pregnancy,” Zernicka-Goetz says. “If it goes wrong, the pregnancy will fail.”
The researchers mimicked natural processes in the lab by guiding three types of stem cells found in early mammalian development to the point where they start interacting. Inducing the expression of a particular set of genes and establishing a unique environment got them to “talk” to each other.
The model copies the stages of natural mouse embryo development that take place up to eight-and-a-half days after fertilization. Over the past decade, Prof. Zernicka-Goetz’s group has been studying these initial stages in order to understand why pregnancies sometimes go wrong.
“The stem cell embryo model is important because it gives us accessibility to the developing structure at a stage that is normally hidden from us due to the implantation of the tiny embryo into the mother’s womb,” the study author explains. “This accessibility allows us to manipulate genes to understand their developmental roles in a model experimental system.”
Study authors sparked development of their synthetic embryo by weaving together different cultured stem cells. They represented each of the three types of tissue in the right proportions and environment to promote growth and communication. After self-assembling into an embryo, the researchers found they signaled chemically, mechanistically, and through touch.
“This period of human life is so mysterious, so to be able to see how it happens in a dish—to have access to these individual stem cells, to understand why so many pregnancies fail and how we might be able to prevent that from happening—is quite special,” Zernicka-Goetz adds. “We looked at the dialogue that has to happen between the different types of stem cells at that time—we’ve shown how it occurs and how it can go wrong.”
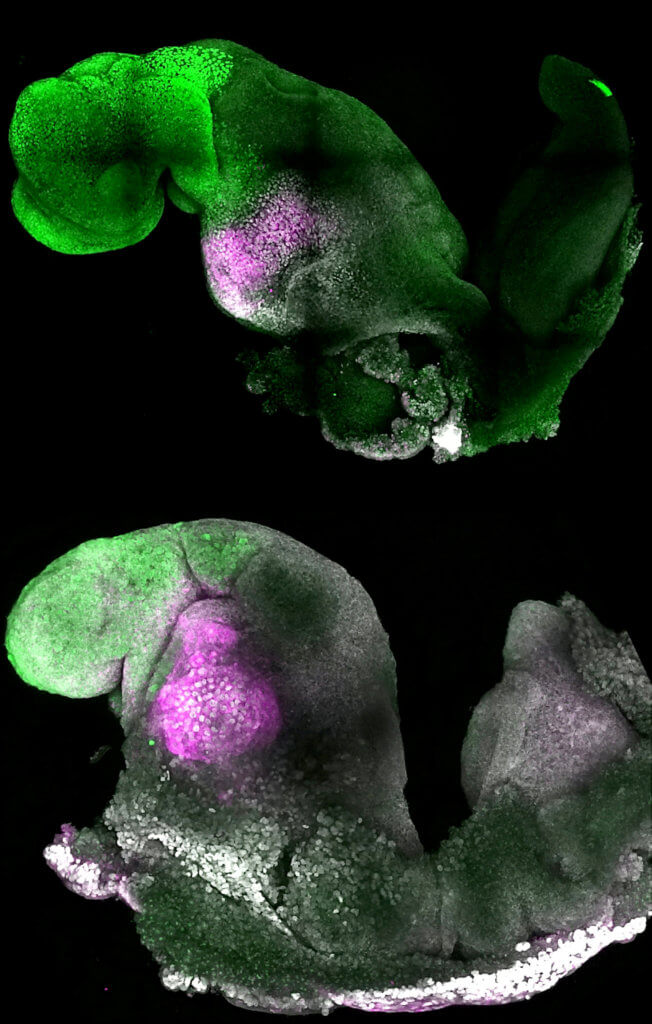
A major advance is the ability to generate the entire brain, in particular the anterior — a big goal in the development of synthetic embryos. In Prof. Zernicka-Goetz’s system, this part requires messages from one of the extra-embryonic tissues to be able to develop.
“This opens new possibilities to study the mechanisms of neurodevelopment in an experimental model,” Zernicka-Goetz explains.
“In fact, we demonstrate the proof of this principle in the paper by knocking out a gene already known to be essential for formation of the neural tube, precursor of the nervous system, and for brain and eye development. In the absence of this gene, the synthetic embryos show exactly the known defects in brain development as in an animal carrying this mutation. This means we can begin to apply this kind of approach to the many genes with unknown function in brain development.”
There could be legal issues for human experiments
The researchers are now developing similar human models with the potential to be directed towards the generation of specific organ types to understand mechanisms behind crucial processes that would be otherwise impossible to study in real embryos.
Currently, U.K. law permits human embryos to be studied in the laboratory only up to the 14th day of development. The findings, published in the journal Nature, could lead to the development of synthetic organs for patients awaiting transplants.
“There are so many people around the world who wait for years for organ transplants,” Zernicka-Goetz concludes. “What makes our work so exciting is that the knowledge coming out of it could be used to grow correct synthetic human organs to save lives that are currently lost. It should also be possible to affect and heal adult organs by using the knowledge we have on how they are made.”
The creation of “synthetic” human embryos is outside of the legal framework of the U.K.’s Human Fertilization and Embryology Act. However, it would be unlawful to use them to establish a pregnancy in a woman, because they are not classed as “permitted embryos.”
South West News Service writer Mark Waghorn contributed to this report.