BALTIMORE — People are becoming more and more aquatinted with the dangers of viruses and how easily these pathogens spread. Now, concerning new findings by a team from the University of Maryland Baltimore County finds certain viruses can actually “monitor” their environment.
Study authors say viruses gather information on their environments, and then use that insight to “decide” the best time to remain dormant and sit tight inside a host, or when is the optimal time to multiply and burst out, killing the host cell. These findings may hold major implications for antiviral drug development moving forward.
The revelation that many viruses are capable of sensing their environment, which includes elements produced by the host, adds “another layer of complexity to the viral-host interaction,” says senior study author Ivan Erill, professor of biological sciences, in a university release.
Right now, Prof. Erill says viruses are exploiting that ability to their benefit. But in the future, “we could exploit it to their detriment.”
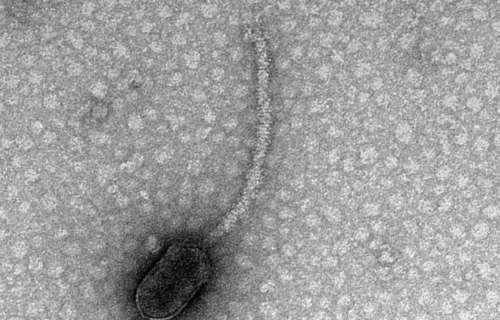
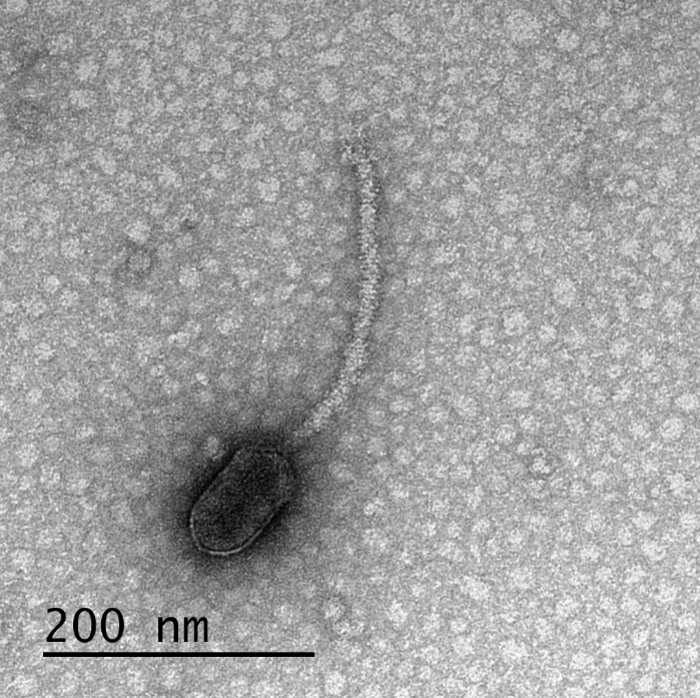
This project focused primarily on bacteriophages, or viruses known to infect bacteria specifically. Bacteriophages are often called “phages.” The phages used for this study could only infect host cells if the bacterial cells featured special appendages called pili and flagella. These appendages help the bacteria move and mate. Bacteria create a protein called CtrA that is responsible for controlling when the appendages are created. This latest research has discovered that numerous appendage-dependent phages display patterns in their DNA where the CtrA protein can attach (binding sites). Prof. Erill explains a phage having a binding site for a protein produced by its host is unusual.
Evolution is upgrading the monitoring process
Through a detailed genomic analysis, study authors also noted the surprising observation that these binding sites don’t appear to be unique to a single phage (or even a single group of phages for that matter). Various different phages boasted CtrA binding sites, but every single one required the host to have either a pili and/or flagella to begin the infection process. Study authors say this can’t be a coincidence.
The ability to monitor CtrA levels “has been invented multiple times throughout evolution by different phages that infect different bacteria,” Prof. Erill notes. When two distantly related species demonstrate a similar trait, it’s referred to as convergent evolution. This observation usually suggests the trait is definitely useful.
The phage containing CtrA binding sites identified by researchers infects a specific group of bacteria called Caulobacterales, which are an especially well-studied class of bacteria.
Why are they so well known?
Caulobacterales exist in two forms; a “swarmer” form that swims around freely, and a “stalked” form that usually attaches to a surface. Importantly, while swarmers typically have pili/flagella, the stalks do not. Among these bacteria, CtrA is also known to regulate the cell cycle, which determines if a given cell will divide evenly into two more of the same cell variety or divide in an asymmetric manner to create one swarmer and one stalk cell.
Since phages can only infect swarmer cells, it makes sense for them to only burst out of their host when there are plenty of swarmer cells available to infect. Most of the time, Caulobacterales live in nutrient-poor environments, and tend to be very spread out. “But when they find a good pocket of microhabitat, they become stalked cells and proliferate,” Prof. Erill adds, eventually leading to large quantities of swarmer cells.
“We hypothesize the phages are monitoring CtrA levels, which go up and down during the life cycle of the cells, to figure out when the swarmer cell is becoming a stalk cell and becoming a factory of swarmers,” the researcher continues, “and at that point, they burst the cell, because there are going to be many swarmers nearby to infect.”
‘Phages are listening in on what’s going on’
The researchers add that the typical methods employed to prove such a hypothesis are quite labor-intensive and extremely difficult. So, that wasn’t part of this latest paper, although Prof. Erill and colleagues hope to one day address that question. However, study authors don’t see any other plausible explanation for the observed proliferation of CtrA binding sites across so many different phages (all of which require pili/flagella to facilitate infection). Moreover, they note the implications for viruses that infect other organisms, even humans, are potentially of serious interest.
“Everything that we know about phages, every single evolutionary strategy they have developed, has been shown to translate to viruses that infect plants and animals,” Prof. Erill says. “It’s almost a given. So if phages are listening in on their hosts, the viruses that affect humans are bound to be doing the same.”
While there are a few other documented examples of phages “monitoring” their environment in various ways, none feature as many different phages employing the same strategy against so many bacterial hosts as this latest work.
This new study is the “first broad scope demonstration that phages are listening in on what’s going on in the cell, in this case, in terms of cell development,” Prof. Erill adds.
Still, he predicts even more examples are on the way. Members of his team are already looking for receptors for other bacterial regulatory molecules in phages – and finding some!
Viruses are making ‘decisions’
Prof. Erill says the key takeaway from this study is that “the virus is using cellular intel to make decisions,” he explains, “and if it’s happening in bacteria, it’s almost certainly happening in plants and animals, because if it’s an evolutionary strategy that makes sense, evolution will discover it and exploit it.”
For instance, if an animal virus wants to optimize its strategy for survival and replication, it will probably want to know what kind of tissue it is in, or how robust the host’s immune response is to infection. Study authors hope these discoveries help open up avenues for new therapies.
“If you are developing an antiviral drug, and you know the virus is listening in on a particular signal, then maybe you can fool the virus. We are just starting to realize how actively viruses have eyes on us—how they are monitoring what’s going on around them and making decisions based on that,” Prof. Erill concludes. “It’s fascinating.”
The study is published in the journal Frontiers in Microbiology.