AMSTERDAM, Netherlands — In the book “The Lovely Bones,” Susie Salmon says an icicle is the perfect murder weapon, and that proves to be the case when an icicle falls and kills her murderer while he was out stalking his next victim. One of the unique features of an icicle is the one-centimeter ripples, though there are some questions why they appear in the first place. In a new study, scientists from Netherlands say icicle ripple formation is largely due to the concentration of salt in the frozen ice water.
In nature, water always carries a small amount of salt. With more salt, the more likely you’ll find visible ripples in icicles. The study authors came to this conclusion after looking at the frozen structures created from their very own icicle machine.
“In a large freezer with a window, we spent a few weeks becoming true icicle growth specialists,” says Menno Demmenie, a PhD student at the Institute of Physics and the Van ’t Hoff Institute for Molecular Sciences at the University of Amsterdam, in a media release.
The main issue throughout the experiment was how quickly they needed to add water before making a “nice” icicle. If you poured water too fast, most of the water would fall to the floor of the freezer into a sheet of ice. Going too slow was just as bad. A slow water flow would make the icicle grow upward, like a stalagmite.
If you had any desire to create an icicle, scientists found the optimal rate of growth happens with an inflow of 60 milliliters per hour, at 5°F.
“This is comparable to a faucet dripping roughly once every three seconds,” describes Demmenie.
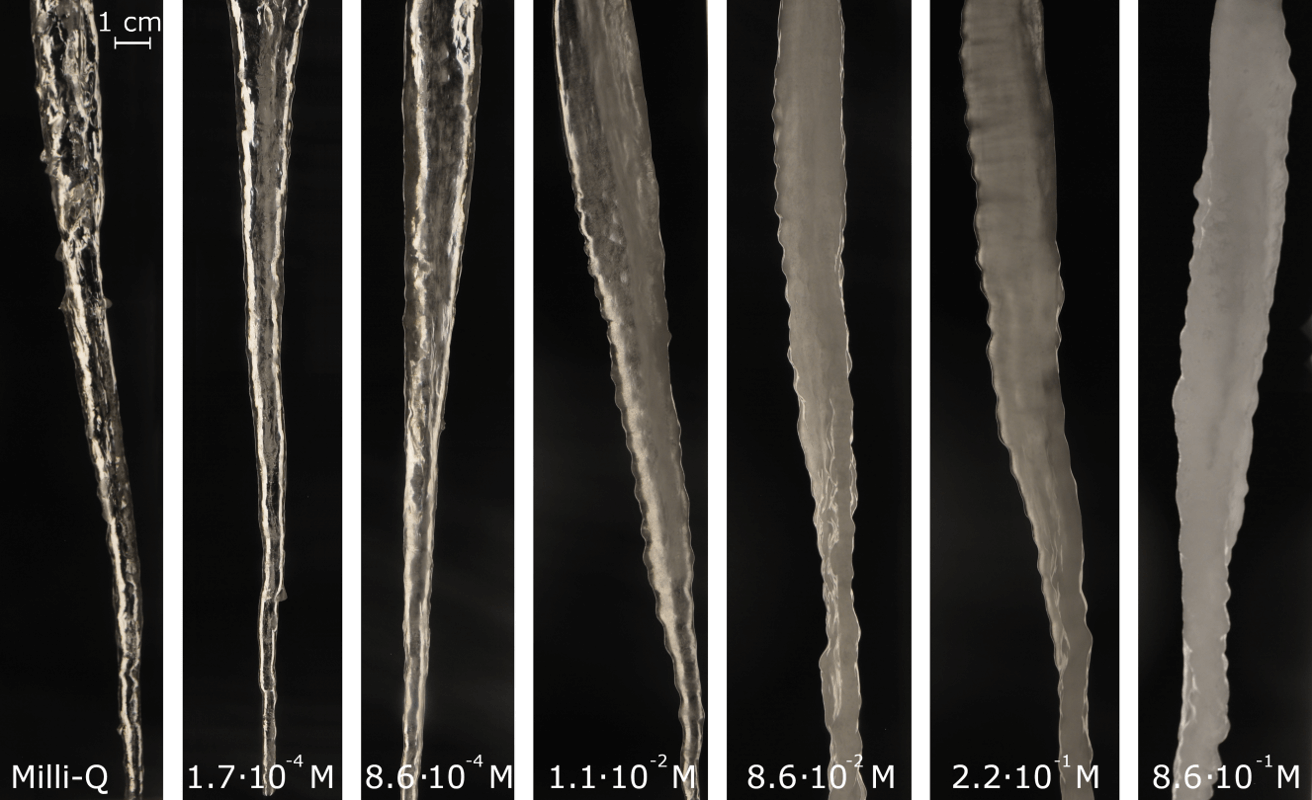
Once they figured out how to make nicely shaped icicles, the researchers used the machine to create multiple icicles under different circumstances and with different types of water. The results show the most important factor in icicle ripple formation is the salt concentration in the water. Icicles made with pure water made less visible ripples than icicles created from salty water.
The researchers realized salt was the main component to rippled icicles by looking at their molecular composition. When the water forming around an icicle freezes, salt molecules are pushed out of the ice as “impurities.” The banished salt molecules take refuge in a thin layer of liquid water on the outside of the icicle.
The team also added a coloring agent to the water used to make icicles, which allowed them to see the flow of water versus where the salt went when frozen. With pure water, the layer of salty liquid water around the icicle was gone and its formation process looked a lot like a dripping candle. When freezing salty water, the icicle developed a thin film of liquid, salty water, and the flow of that water created the ripples. The more salt, the stronger the process to create thicker and more visible ripples.
The findings appear in the journal Physical Review Applied.

