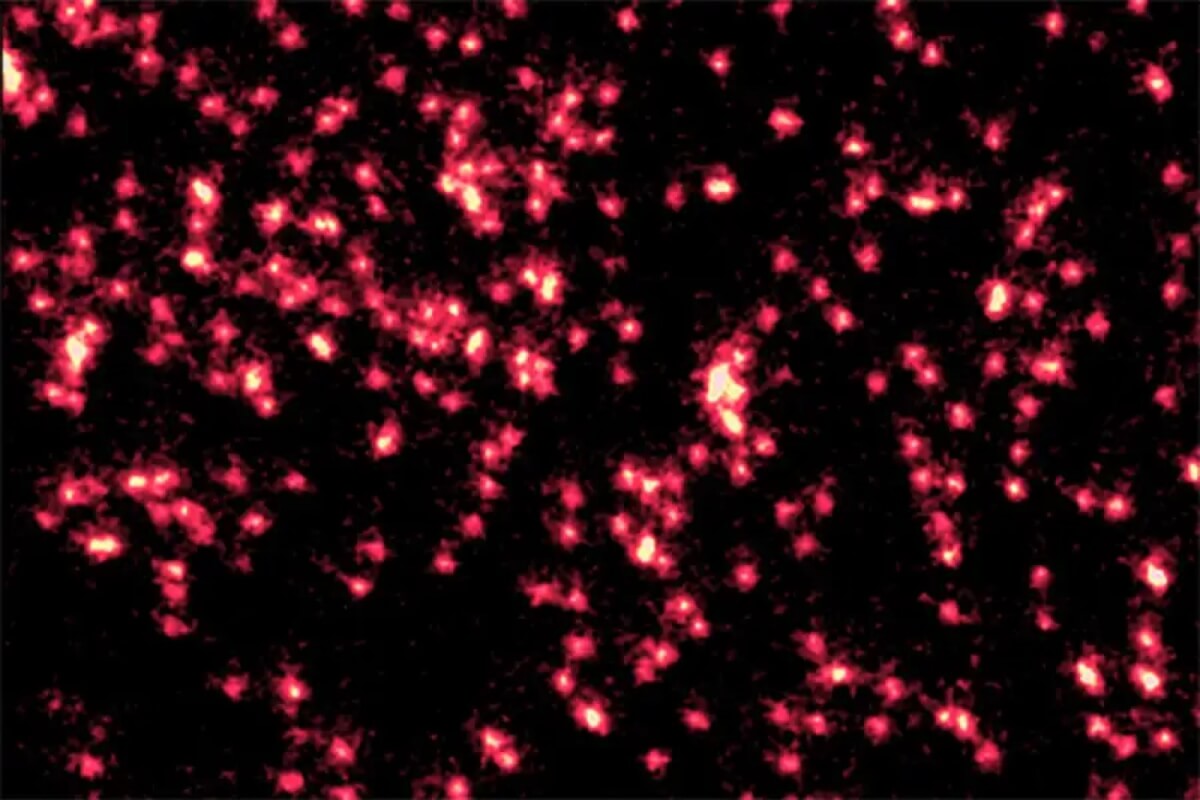
Atoms appear in the clearest image ever behaving like a wave (Credit: Joris Verstraten/arxiv)
PARIS — In the mysterious realm of the quantum world, particles behave in ways that seem to defy our everyday intuition. One of the most fundamental and mind-bending aspects of quantum mechanics is the idea that tiny objects like atoms or electrons can behave as both particles and waves at the same time. This strange “wave-particle duality” has been demonstrated in various experiments over the past century. Now, physicists have managed to capture the first-ever images of individual atoms’ “wave packets” as they expand and evolve in space and time.
To understand what a wave packet is — the mathematical description of their wavelike properties — let’s take a step back and consider what it means for a particle to behave like a wave. In classical physics, a particle has a definite position and velocity at any given moment. But in quantum mechanics, particles are instead described by a “wavefunction” – a complex mathematical object that encodes the probability of finding the particle at any particular location. The wavefunction is often represented visually as a series of peaks and troughs, similar to a water wave or a vibrating guitar string.
However, unlike a water wave, which is spread out over a large area, a quantum wavefunction can be localized to a tiny region of space, forming what’s known as a wave packet. You can think of a wave packet as a small “bundle” of the particle’s wavefunction, representing the area where the particle is most likely to be found. As time passes, this wave packet will naturally spread out and expand, a process known as dispersion. It’s this expansion of atomic wave packets that the researchers have now managed to directly image.
The team, based at the Laboratoire Kastler Brossel in France and publishing their work in arXiv, began by using lasers to trap individual atoms of lithium-6 in an optical lattice — essentially an egg-carton-like structure made of light, with each “dip” in the lattice acting as a tiny trap for a single atom. They cooled the atoms to extremely low temperatures, close to absolute zero, causing each atom’s wave packet to contract and settle into the lowest energy “ground state” of its lattice site.
The researchers then carefully released the atoms from the optical lattice, allowing their wave packets to expand freely in two dimensions, like ripples on a pond. After letting the wave packets evolve for a specific amount of time (ranging from microseconds to tens of microseconds), they rapidly turned the lattice back on, recapturing or “projecting” each atom onto a single lattice site. By repeating this preparation, release, and recapture process many times and imaging the final position of the atoms in the lattice after each run, the team built up a statistical picture or “histogram” of the most likely positions of the atoms. This histogram directly reflects the probability distribution of each atom’s wave packet at a given snapshot in time.

The images reveal the wavelike nature of the atoms in stunning detail, showing the wave packets as fuzzy, concentric rings that expand outward with time, just as ripples spread out on a pond. By tracking how the width of the wave packets increased linearly with time, the researchers confirmed that the atoms’ behavior matched the predictions of quantum theory with remarkable precision.
One key challenge the team had to overcome was how to deal with the transition from the quantum world to the classical world when “catching” the atoms in the lattice for imaging. They had to carefully optimize the speed at which the lattice was turned back on – too slow, and the atoms would continue to move and disperse during the recapture process, blurring out the image. But flick the lattice on too abruptly, and you’d create unwanted excitations or “sloshing” of the atoms in their traps, also degrading the final snapshot. The sweet spot, they found, was a lattice ramp-up time of around 2-5 microseconds.
While the images showcase the wave-particle duality of individual atoms in unprecedented detail, the researchers say the real excitement is in the future potential of this imaging technique. By opening the door to visualizing quantum wave packets in situ (in its natural setting), this work sets the stage for probing far more complex quantum phenomena in the future.
One tantalizing prospect is using the same method to directly image the wave packets of interacting atoms – for example, in a quantum fluid where the atoms are strongly entangled with one another. This could allow physicists to capture snapshots of exotic states of matter, such as supersolids or fractional quantum Hall fluids, which are extremely challenging to characterize through other means.
The ability to image wave packets could also lead to new insights in the study of quantum information and computing, where the delicate wave-like states of atoms, electrons, or photons can be harnessed to perform complex calculations that would be intractable for classical computers. Being able to visualize how these “qubits” evolve and interact in real-time could guide efforts to build more robust quantum devices.
From a fundamental physics perspective, the team’s approach represents a powerful new tool for exploring the boundary between the quantum and classical realms. As the wave packets expand and grow, they start to behave more and more like classical objects. Tracing this transition in detail could shed light on the still-murky process of how quantum behavior gives way to the classical physics of our everyday experience.
In the words of the famous physicist Richard Feynman, “Nature isn’t classical, dammit, and if you want to make a simulation of nature, you’d better make it quantum mechanical.” With their pioneering snapshots of single-atom wave mechanics in action, the Laboratoire Kastler Brossel team has given us a breathtaking new window into the quantum world, bringing us one step closer to Feynman’s vision of seeing nature as it really is, in all its strange and wonderful glory.










