RESTON, Va. — A promising new treatment for a deadly blood cancer essentially cures the disease and wipes out tumors in just two days. Researchers working with the Society of Nuclear Medicine and Molecular Imaging say the new therapy could be a game-changing step in the treatment of non-Hodgkin lymphoma. In experiments with mice, just one dose of the radioimmunotherapy [177Lu]Lu-ofatumumab eliminated tumors in hours and kept the cancer from returning.
According to the American Cancer Society, doctors will diagnose roughly 80,500 new cases of non-Hodgkin lymphoma (NHL) throughout the United States in 2023 and 20,100 people will die from the disease. NHL is a common blood malignancy. Currently, standard treatments involve chemotherapy and immunotherapy targeting the CD20 protein — which non-Hodgkin lymphoma cells express frequently.
“Although this chemotherapy with immunotherapy combination is usually initially effective, many patients don’t respond or relapse, so we need improved therapies,” notes Richard Wahl, MD, the Elizabeth E. Mallinckrodt Professor and director of Mallinckrodt Institute of Radiology at Washington University School of Medicine in St. Louis, in a media release.
To put the new drug’s effectiveness into context, mice taking [177Lu]Lu-ofatumumab survived for more than 221 days — the endpoint of the preclinical trial. Mice taking other non-Hodgkin lymphoma treatments only survived for fewer than 60 days and untreated mice died after just 19 days.
What makes the new treatment different from standard cancer drugs?
Researchers labeled ofatumumab, a recently developed anti-CD20 fully human antibody, with 177Lu, a common therapeutic radioisotope which kills cancer cells. In lab experiments, the team examined the characteristics of [177Lu]Lu-ofatumumab, estimated how much human patients would need in an injection, and evaluated its effectiveness against non-Hodgkin lymphoma using mice carrying the disease.
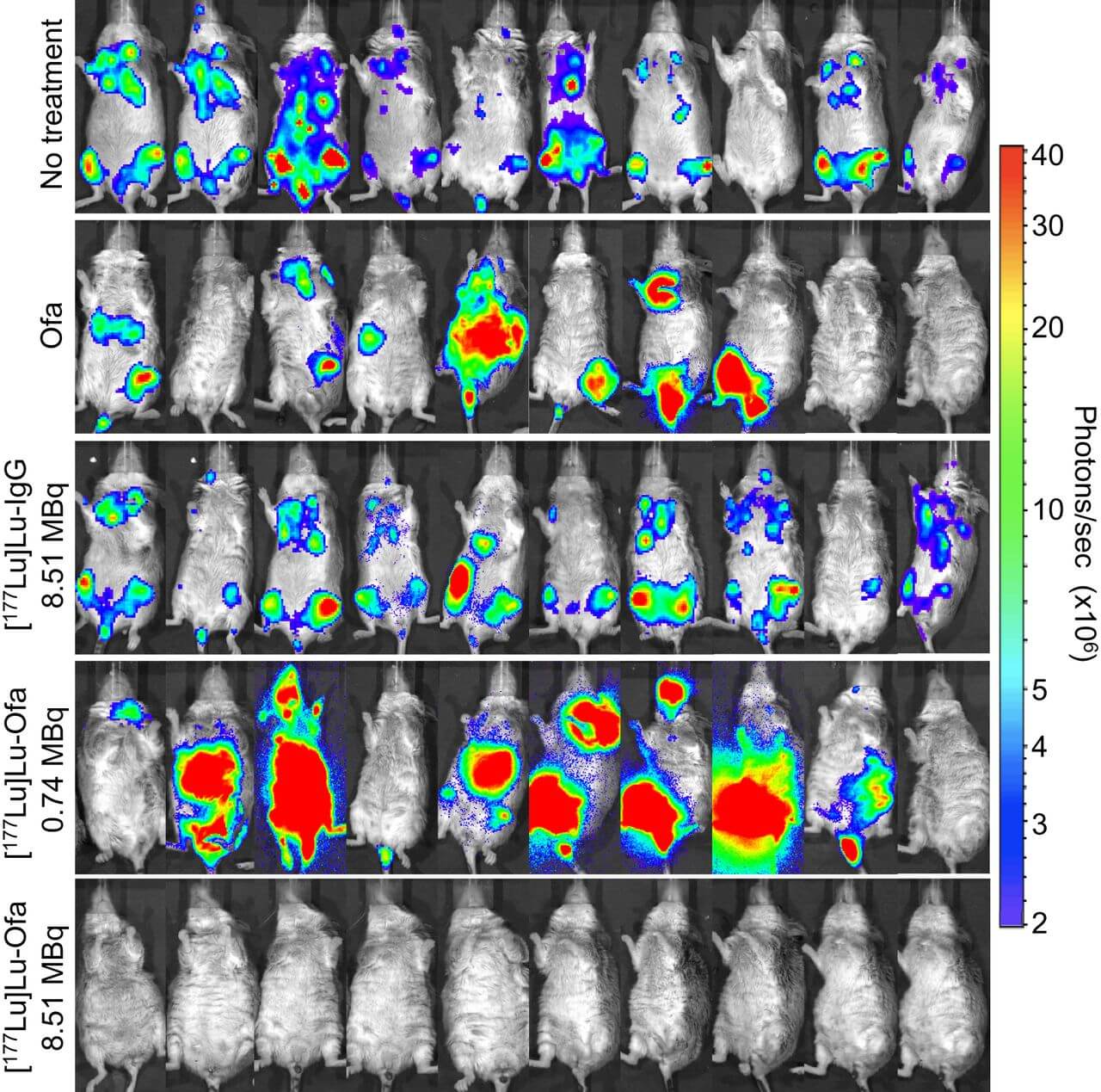
During the preclinical trial, researchers treated mice with human B cell lymphoma with either unlabeled ofatumumab, 8.51 MBq of [177Lu]Lu-IgG, 0.74 MBq or 8.51 MBq of [177Lu]Lu-ofatumumab, or didn’t treat them at all. Results show 8.51 MBq of [177Lu]Lu-ofatumumab produced the best results, helping the mice to survive throughout the entire trial period — essentially curing them of non-Hodgkin lymphoma.
“What’s more, in mice treated with 8.51 MBq of [177Lu]Lu-ofatumumab, detectable tumors were eliminated completely within two days. Mice treated with the other therapies or left untreated, on the other hand, continued to show tumor cells present,” Wahl reports.
Mice taking unlabeled ofatumumab survived for 46 days on average, while those taking [177Lu]Lu-IgG and 0.74 MBq of [177Lu]Lu-ofatumumab survived for 25 and 59 days, respectively. As for making the new drug, study authors say they were able to produce [177Lu]Lu-ofatumumab with both high yield and high purity. Overall, they believe their results will translate over to human patients — making a cure for non-Hodgkin lymphoma a real possibility in the near future.
“The excellent therapeutic results in this animal model of human B cell lymphoma suggest that this curative treatment should be tested in humans with non-Hodgkin lymphoma,” Wahl concludes. “If testing is successful in humans, this would represent an excellent new treatment option for patients with this disease.”
The findings appear in The Journal of Nuclear Medicine.

