SEATTLE, Wash. — For anyone that has had to wait or had a loved one wait on an organ donor list, they know how precious a transplant is. Now, researchers from the University of Washington say a new technique could help create lab-grown, fully functional organs ready for transplant.
While scientifically-designed human organs may sound like science fiction, many labs across the world are hoping to one day make them reality. Before an organ can be made in a lab however, researchers say there are two major hurdles to overcome. The first is creating a biologically compatible 3D scaffold in which cells can grow. The second is triggering cells to grow into the desired organ or tissue.
Some scientists have been able to create lab-grown skin grafts to help burn victims, but fully functioning organs are a taller order. The UW team has developed a technique to modify naturally occurring biological polymers that affect cell behavior.
Using lasers to replicate new organs
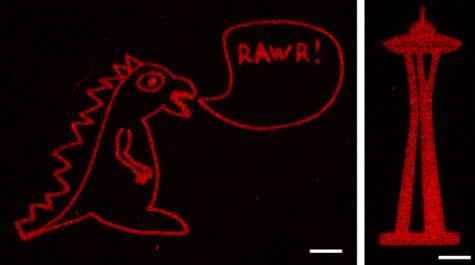
Their approach uses a near infrared laser to trigger proteins to stick to a scaffold made from collagen. The researchers were able to create any shape they wanted from the laser-created proteins, including Seattle’s Space Needle, a monster, and the 3D layout of the human heart.
The scientists say this technique is a major step in controlling cell function and creating engineered organs. The tethered proteins were fully functional, delivering the desired signals to cells.
Rat liver cells, when loaded onto the collagen hydrogel, promoted cell growth and showed signs of DNA replication and cell division. The experiments with different biological scaffolds and protein signals indicated the approach could work for almost any type of protein and biomaterial system.
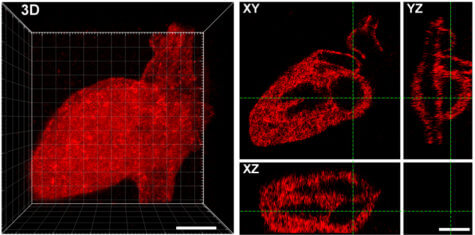
“These methods could form the basis of biologically based scaffolds that might one day make functional laboratory-grown tissues a reality,” says Dr. Cole DeForest, an associate professor of chemical engineering and bioengineering, in a university release.
“This approach provides us with the opportunities we’ve been waiting for to exert greater control over cell function and fate in naturally derived biomaterials — not just in three-dimensional space but also over time. Moreover, it makes use of exceptionally precise photochemistries that can be controlled in 4D while uniquely preserving protein function and bioactivity.”
The findings appear in the Proceedings of the National Academy of Sciences.
SWNS writer Joe Morgan contributed to this report.