CAMBRIDGE, United Kingdom — There have been numerous studies examining the origins of life on Earth. One says that a concoction of chemical ingredients found in everyday items like margaritas, vinegar, and even the sting of an ant is the secret recipe of life. Another states that hot springs were potentially the birthplace of life billions of years ago. Even the Bible has put forward its own version of how Earth was created — however, it hasn’t been scientifically proven. Now, a new study from the University of Cambridge researchers suggests that the key ingredients — nitriles and isonitriles — may have bubbled up from graphite-rich volcanic vents on the newly formed Earth.
The findings, published in the journal Life, lay out a step-by-step scenario for how these crucial compounds could have formed in the hellish conditions of the planet’s youth when massive impacts were frequent.
“A big part of life is simplicity,” says study co-author Dr. Paul Rimmer, assistant professor of experimental astrophysics at the University of Cambridge’s Cavendish Laboratory, in a media release. “It’s order. It’s coming up with a way to get rid of some of the complexity by controlling what chemistry can happen.”
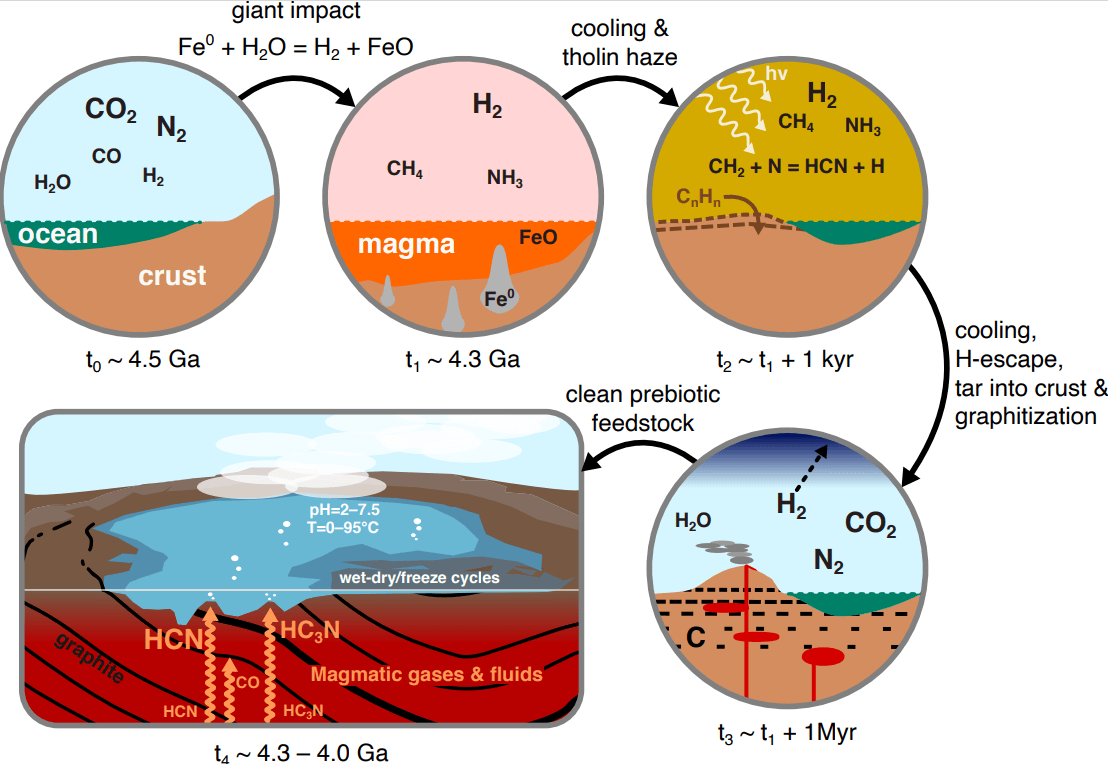
Around 4.3 billion years ago during the Hadean eon — the earliest period in Earth’s history — scientists believe an object the size of the Moon hit Earth, causing the object’s iron to react with water.
“Something the size of the moon hit early Earth, and it would have deposited a large amount of iron and other metals,” notes study co-author Dr. Oliver Shorttle, professor of natural philosophy at the Institute of Astronomy and Department of Earth Sciences in Cambridge.
These giant impacts generated a transient hydrogen-rich atmosphere, which reduced atmospheric carbon. This reduced carbon would condense into thick hazes that rained down onto the hot surface, becoming incorporated into the crust.
When ancient magma oozed up through this graphite-saturated crust, it scrubbed the volcanic gases clean. The leftover gas was purified due to the equilibrium with graphite. This resulted in significant quantities of nitriles and isonitriles relevant for prebiotic chemistry.
Nitriles, like hydrogen cyanide (HCN), and isonitriles, such as methyl isocyanide, are thought to be essential precursors for the synthesis of life’s building blocks — nucleotides, amino acids, and lipids. But cooking up these compounds in a messy primordial environment is tricky.
That’s where graphite comes in. When magma temperatures reach a searing 3,092 degrees Fahrenheit, graphite formation pulls out all the gunk, leaving behind a streamlined cocktail of HCN, cyanoacetylene (HC3N), hydrogen isocyanide (HNC), and not much else.
According to the model, at these temperatures and 100 times atmospheric pressure, HCN concentrations could reach one percent, HC3N around 1 part per million (ppm), and HNC about 0.01 percent. Methyl isocyanide levels are estimated to be in the 1 ppm range.
Cambridge researchers explored a range of temperatures, pressures, and elemental abundances in their calculations. They found that only a Goldilocks set of conditions — graphite saturation, temperatures above 2,700 degrees Fahrenheit, and high pressure — yield the jackpot of prebiotic chemicals.
“Once the iron reacts with the water, a mist forms that would have condensed and mixed with the Earth’s crust. Upon heating, what’s left is, lo and behold, the useful nitrogen containing compounds,” explains Dr. Shorttle.
Go too low in temperature, below 2,300 degrees Fahrenheit, and most of the carbon and nitrogen get locked up in graphite and N2 gas. Dial down the pressure, and cyanoacetylene yields plummet.
The required ultra-high temperatures point to specialized volcanic environments like komatiites — searing hot lava flows found on the early Earth over 3.5 billion years ago.
“Crucially, we know that these rocks only form at scorching temperatures, around 1,700 degrees Celsius! That means the magma would already have been hot enough to heat the tar and create our useful nitriles,” says Dr. Shorttle.
Once spewed out, these volcanic gases could de-gas into surface or shallow hydrothermal vents. There, ultraviolet light may drive further synthesis of key ingredients like RNA nucleotides, while mineral surfaces catalyze their assembly.

The study makes testable predictions that future experiments can interrogate. Does pressure suppress cyanoacetylene formation? Can these compounds survive the quenching process as magma meets water?
Ultimately, unraveling the specifics of this “volcanic recipe” would lend crucial insight into the chemical steps that bridged the gap between geology and biology at life’s dawn. It may even help us identify other worlds where life could arise.
“Though we don’t know for sure that these molecules started out life on Earth, we do know that life’s building blocks must be made from molecules that survived in water,” concludes Dr. Rimmer. “If future experiments show that the nitriles all fall apart, then we’ll have to look for a different way.”
By narrowing the parameter space and proposing tractable mechanisms, research like this brings us closer to understanding one of science’s most enduring enigmas — the origin of life on Earth and beyond. If the secret ingredients really did spew forth from the planet’s fiery depths eons ago, it would be a profound realization. We, and all life as we know it, may be children of volcanoes.
StudyFinds’ Matt Higgins contributed to this report.
