MEDFORD, Mass. — An innovative creation will help transform treating diseases. Scientists at Tufts University and Harvard University’s Wyss Institute developed minuscule biological robots from human cells. Crafted from human tracheal cells, these “Anthrobots” possess the astonishing ability to move across surfaces and have exhibited a remarkable healing effect by prompting neuron growth in damaged lab dish regions.
The Anthrobots, ranging from the width of a human hair to the tip of a sharpened pencil, were designed to self-assemble and have demonstrated an impressive capacity to aid in cell healing. This discovery serves as a crucial stepping stone toward the researchers’ vision of utilizing patient-derived biobots as innovative therapeutic tools for regeneration, healing, and disease treatment.
This breakthrough stems from earlier research conducted by Michael Levin, Vannevar Bush Professor of Biology at Tufts University School of Arts & Sciences, and Josh Bongard at the University of Vermont. They created multicellular biological robots called Xenobots from frog embryo cells, capable of various functions such as navigating, collecting materials, recording information, and even self-replicating for a limited number of cycles. However, it was unclear if these capabilities were exclusive to amphibian embryo-derived cells or if biobots could be formed using cells from other species.
In their latest study, Levin and Tufts PhD student Gizem Gumuskaya discovered that Anthrobots can indeed be crafted from adult human cells without genetic modification, showcasing capabilities surpassing those observed in Xenobots. This finding begins to address a broader question posed by the lab — what rules govern cell assembly and collaboration within the body, and can cells be repurposed into different “body plans” to perform distinct functions?
Gizem Gumuskaya, Tufts University)
Researchers provided human cells, which typically serve mundane functions in the trachea, an opportunity to reprogram and explore new structural and functional tasks. “We wanted to probe what cells can do besides create default features in the body,” says Gumuskaya, who earned a degree in architecture before coming into biology, in a university release. “By reprogramming interactions between cells, new multicellular structures can be created, analogous to the way stone and brick can be arranged into different structural elements like walls, archways or columns.”
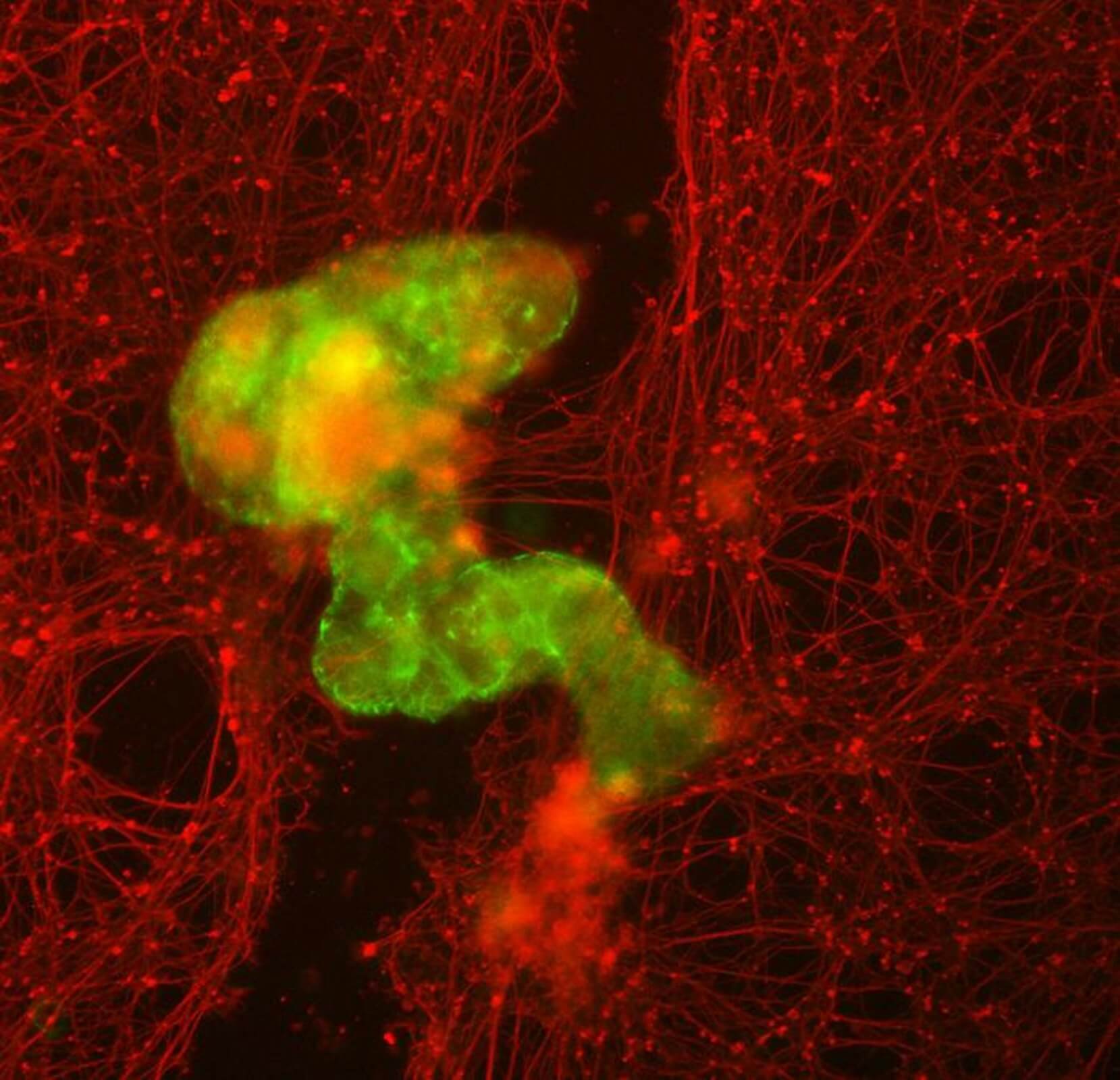
Anthrobots showcased not only the creation of novel multicellular shapes but also the ability to maneuver across a surface covered in human neurons grown in a lab dish, prompting new growth to fill gaps caused by cell layer scratching.
The exact mechanism behind how Anthrobots stimulate neuron growth remains unclear.
“The cellular assemblies we construct in the lab can have capabilities that go beyond what they do in the body,” notes Levin, who also serves as the director of the Allen Discovery Center at Tufts and is an associate faculty member of the Wyss Institute. “It is fascinating and completely unexpected that normal patient tracheal cells, without modifying their DNA, can move on their own and encourage neuron growth across a region of damage. We’re now looking at how the healing mechanism works, and asking what else these constructs can do.”
One of the main advantages of using human cells lies in constructing biobots from a patient’s cells to perform therapeutic tasks without triggering immune responses or requiring immunosuppressants. These Anthrobots naturally break down after a few weeks and can be easily absorbed into the body once their function is complete.
Anthrobots can only survive under specific laboratory conditions, posing no risk of exposure or unintended spread outside the controlled environment. They do not reproduce, have no genetic alterations, and therefore carry no risk of evolving beyond safety measures.
How Anthrobots Are Created
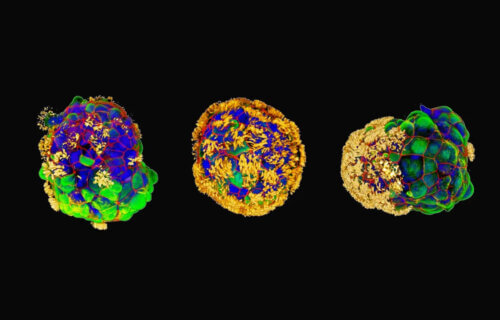
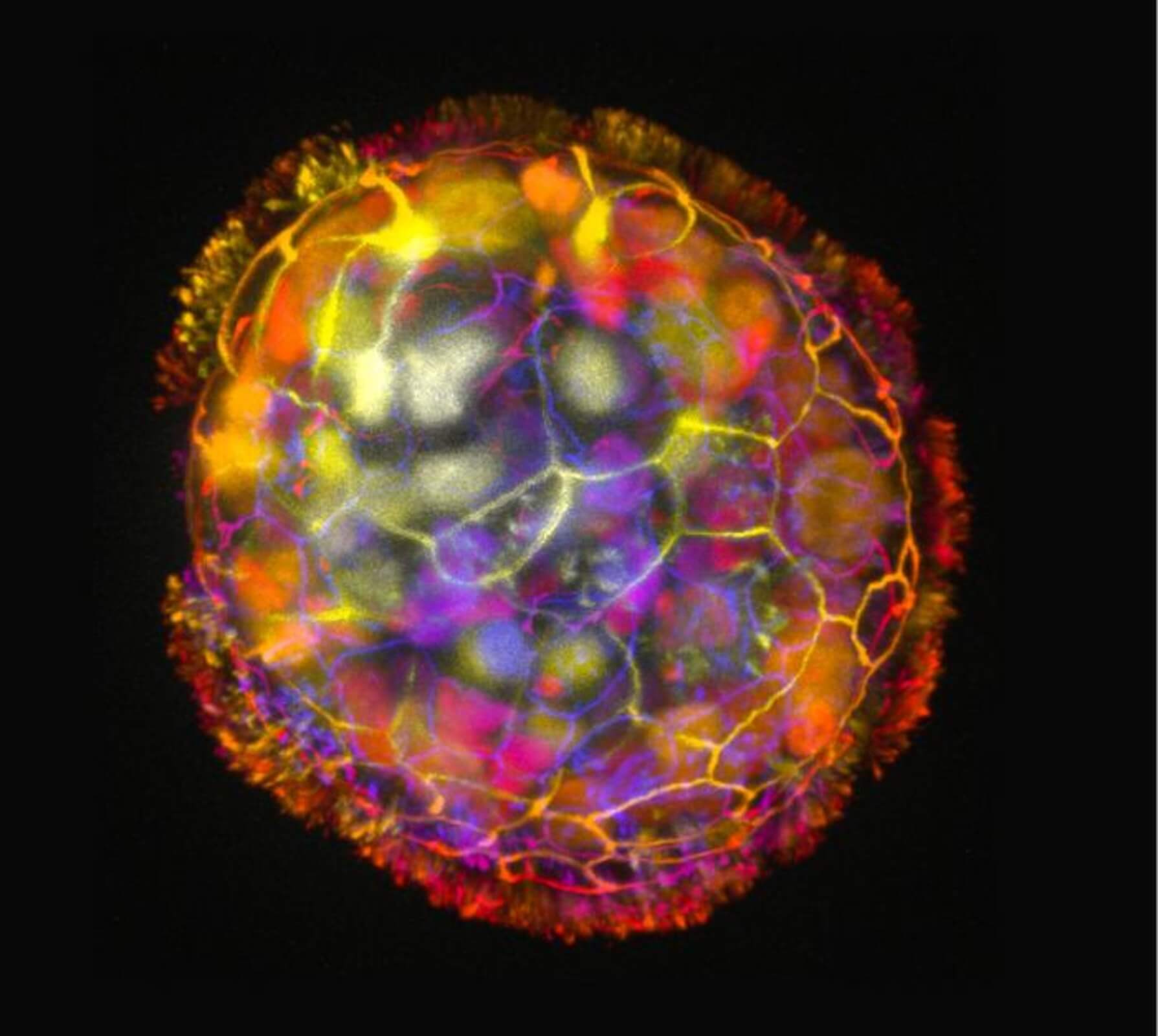
Each Anthrobot originates from a single cell extracted from an adult donor’s tracheal surface cells. These cells possess cilia, hair-like projections that aid in propelling tiny particles out of the air passages of the lung, a process we often experience through coughing or clearing our throats. When grown in a lab, these cells naturally form tiny multicellular spheres known as organoids.
Researchers devised growth conditions that directed cilia to face outward on organoids. Within days, these structures began moving, propelled by the cilia acting like oars. The team observed various shapes and movement types, a groundbreaking feature observed in this biobotics platform.

By characterizing the different types of Anthrobots produced, researchers identified spherical bots fully covered in cilia, irregular or football-shaped bots with patchy cilia coverage, and those covered with cilia only on one side. These bots ranged from 30 to 500 micrometers, bridging the gap between nanotechnology and larger engineered devices.
Some Anthrobots moved in straight lines or tight circles, while others combined these movements or exhibited stationary wiggling. These structures typically survived for 45-60 days under lab conditions before biodegrading naturally.
Potential for Therapeutic Applications
Levin and Gumuskaya envision therapeutic applications for Anthrobots and conducted tests to assess their wound-healing capabilities. Using a model involving a layer of human neurons, they simulated a ‘wound’ by scratching the cell layer. Anthrobots, forming a dense ‘superbot’ cluster, triggered significant neural regrowth, bridging the gap created in the cell layer.
In what surprised scientists, unmodified Anthrobots facilitated substantial regrowth without genetic modifications, showcasing efficient healing of live neural tissue in the lab dish.

Looking ahead, researchers anticipate broader applications for Anthrobots, including clearing plaque buildup in atherosclerosis patients’ arteries, repairing spinal cord or retinal nerve damage, identifying bacteria or cancer cells, or delivering targeted drugs.
Pioneering New Blueprints and Restoring Old Ones
Gumuskaya highlighted the inherent ability of cells to self-assemble into larger structures.
“The cells can form layers, fold, make spheres, sort and separate themselves by type, fuse together, or even move,” explains Gumuskaya. “Two important differences from inanimate bricks are that cells can communicate with each other and create these structures dynamically, and each cell is programmed with many functions, like movement, secretion of molecules, detection of signals and more. We are just figuring out how to combine these elements to create new biological body plans and functions — different than those found in nature.”
This flexible cellular assembly not only aids in constructing biobots but also holds promise in understanding natural body plan assembly, genome-environment interactions in tissue and organ creation, and regenerative treatments.
The study is published in the journal Advanced Science.