PHILADELPHIA — South Korean scientists have created a simple, noninvasive test that may lead to an early diagnosis of bladder cancer. This tool could decrease the need for invasive diagnostic procedures and thereby lessen the financial strain on the healthcare system.
Bladder cancer is a frighteningly common disease, ranking as the sixth most diagnosed cancer worldwide. It often first reveals itself through the appearance of blood in the urine, a symptom known as hematuria. However, hematuria is not a surefire sign of bladder cancer – in fact, only about 10% of people with visible blood in their urine and 2-5% of those with microscopic amounts of blood actually have bladder cancer.
While the U.S. Food and Drug Administration (FDA) has approved several urine-based tests for potential early detection, they haven’t been successfully used for early bladder cancer diagnosis.
So what’s a person with hematuria to do? Currently, the gold standard for diagnosing bladder cancer is a procedure called a cystoscopy. In this test, a thin, flexible tube with a light and camera on the end is inserted through the urethra and into the bladder, allowing the doctor to visually examine the bladder lining for any suspicious growths or lesions. While effective, cystoscopies are invasive, uncomfortable, and costly.
This is where a group of innovative researchers comes in. Led by researchers Dr. Sungwhan An, CEO and Scientific Director of Genomictree, Inc., and Dr. Ju Hyun Shin, of the Department of Urology at Chungnam National University College of Medicine, this team has developed a new urine-based test that could help identify bladder cancer in patients with hematuria, potentially reducing the need for cystoscopies. Their research is published in the Journal of Molecular Diagnostics.
“Diagnosing bladder cancer at an early stage is critical and not only can increase patient survival rates but can also contribute to reducing healthcare costs,” the authors explain in a media release. “Current guidelines recommend cystoscopy and imaging examinations for almost all patients presenting with hematuria for initial diagnosis of bladder cancer, but it is invasive, inconvenient, economically burdensome for patients, and frequently fails to detect early-stage bladder cancer. There is therefore an urgent need for a sensitive and precise technique to diagnose early bladder cancer effectively among patients with hematuria.”
Their test looks for a specific epigenetic change in cells shed from the bladder lining into the urine. Epigenetic changes are modifications to DNA that don’t alter the DNA sequence itself, but rather influence which genes are turned on or off. One common epigenetic change in cancer is called DNA methylation, where methyl groups are added to certain sections of DNA, often silencing tumor suppressor genes that would normally help prevent cancer growth.
The researchers focused on methylation of a gene called PENK. Previous studies had shown that PENK methylation occurs frequently in bladder cancer cells. However, detecting these methylated PENK genes in urine can be tricky, especially in early stage cancers that shed very few cells.
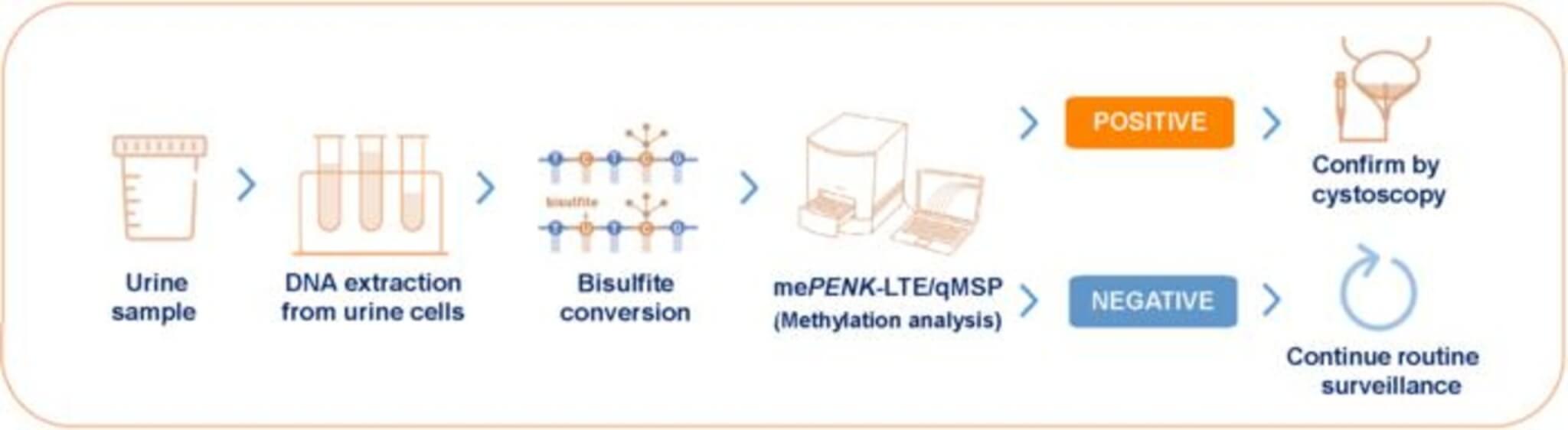
To overcome this challenge, the team developed a highly sensitive two-step process they call mePENK-LTE/qMSP. In the first step, they use a technique called linear target enrichment (LTE) to amplify and enrich the methylated PENK DNA from the urine sample. Then, they use quantitative methylation-specific PCR (qMSP) to precisely measure the amount of methylated PENK present.
In a case-control study involving 175 patients with confirmed bladder cancer and 143 controls with hematuria from other causes, the mePENK-LTE/qMSP test achieved an impressive overall sensitivity of 86.9% for detecting bladder cancer, with a specificity of 91.6%. Sensitivity refers to the test’s ability to correctly identify those with the disease, while specificity refers to its ability to correctly identify those without the disease.
The researchers then validated their test in a prospective study of 366 patients presenting with hematuria who were all scheduled for cystoscopy. In this real-world setting, the test maintained a high sensitivity of 84.2% and a specificity of 95.7%. Notably, the test was particularly good at detecting high-grade and more advanced-stage tumors, with a sensitivity of 92.3% for these cases.
Based on a bladder cancer prevalence of 10% among patients with hematuria, the test’s negative predictive value was 98.2%. This means that if the test comes back negative, there’s a 98.2% chance that the patient does not have bladder cancer. Such a high negative predictive value suggests that the test could be used to rule out bladder cancer in many patients with hematuria, potentially allowing them to safely avoid a cystoscopy.
Of course, no test is perfect, and false positives can occur. The researchers note that in patients with other genitourinary cancers like prostate or kidney cancer, the test can sometimes come back positive even in the absence of bladder cancer. Therefore, if the mePENK test is positive but the cystoscopy is negative, additional imaging may be warranted to check for these other cancers.
The researchers also compared their test to another commercially available urine test for bladder cancer called NMP22. In a head-to-head comparison, the mePENK-LTE/qMSP test outperformed NMP22, with a significantly higher sensitivity and specificity.
While these results are promising, the researchers acknowledge some limitations of their study. The patients were all from a single center, and information on potentially relevant factors like smoking history was not available. Additionally, the number of bladder cancer cases in the prospective validation study was relatively small. Larger, multi-center studies will be needed to further validate the test’s performance.
Nonetheless, this study represents an exciting step forward in the quest for non-invasive methods to detect bladder cancer early. By identifying bladder cancer through a simple urine test, many patients with hematuria could potentially be spared the discomfort and expense of a cystoscopy. Moreover, the test’s strong performance in detecting high-grade and advanced tumors suggests it could help catch aggressive cancers early, when they are most treatable.
As the researchers continue to refine and validate their test, it’s easy to envision a future where a trip to the bathroom could provide a wealth of information about your bladder health. For the millions of people worldwide affected by bladder cancer, that’s an exciting prospect indeed.

As a bladder cancer patient, saying that a cystoscopy is “uncomfortable” is an understatement! I suppose for manly men, it may be uncomfortable. But for me, the procedure is downright p-a-i-n.
Also, not stated is the residual “discomfort” (i.e. pain) of the urethra. The tube that is inserted bruises the urethra, so that there is a healing time one must allow for after the cystoscopy. The more cystoscopies, the longer the urethra”discomfort” (i.e. pain) lasts.
And then there is the voiding frequency. It changes from your normal to more frequent; oftentimes far more frequent than you want.
So as the article says, anything to avoid the cystoscopy will be greatly appreciated.