PITTSBURGH — A novel study suggests that a simple blood test might identify healthy senior citizens who are likely to develop Alzheimer’s disease. Despite extensive research, medical practitioners have struggled to understand why some individuals develop this debilitating neurodegenerative condition, characterized by progressive memory loss and confusion, while others do not. Intriguingly, many individuals whose brains display a significant buildup of toxic amyloid aggregates — a common indicator of Alzheimer’s pathology — never progress to develop dementia.
A team of scientists at the University of Pittsburgh School of Medicine in the United States seems to have found an answer. According to their groundbreaking research, published in the journal Nature Medicine, star-shaped brain cells, known as astrocytes, play a pivotal role in the progression of Alzheimer’s disease.
The researchers conducted blood tests on over 1,000 cognitively unimpaired elderly people, some with amyloid pathology and some without. The team discovered that only those individuals exhibiting both amyloid buildup and abnormal astrocyte activation markers in their blood would eventually progress to symptomatic Alzheimer’s.
This critical discovery holds substantial implications for drug development aimed at halting the disease’s progression.
“Our study argues that testing for the presence of brain amyloid along with blood biomarkers of astrocyte reactivity is the optimal screening to identify patients who are most at risk for progressing to Alzheimer’s disease,” says senior author Tharick Pascoal, M.D., Ph.D., associate professor of psychiatry and neurology at Pitt, in a media release. “This puts astrocytes at the center as key regulators of disease progression, challenging the notion that amyloid is enough to trigger Alzheimer’s disease.”
What’s really causing the world’s most common form of dementia?
Historically, scientists believed that the accumulation of amyloid plaques — protein aggregates lodged between the brain’s nerve cells — and disordered protein fibers, known as tau tangles, within neurons were direct culprits of Alzheimer’s disease. This belief led drug manufacturers to heavily invest in molecules targeting amyloid and tau, disregarding the contributions of other brain processes, such as the neuroimmune system.
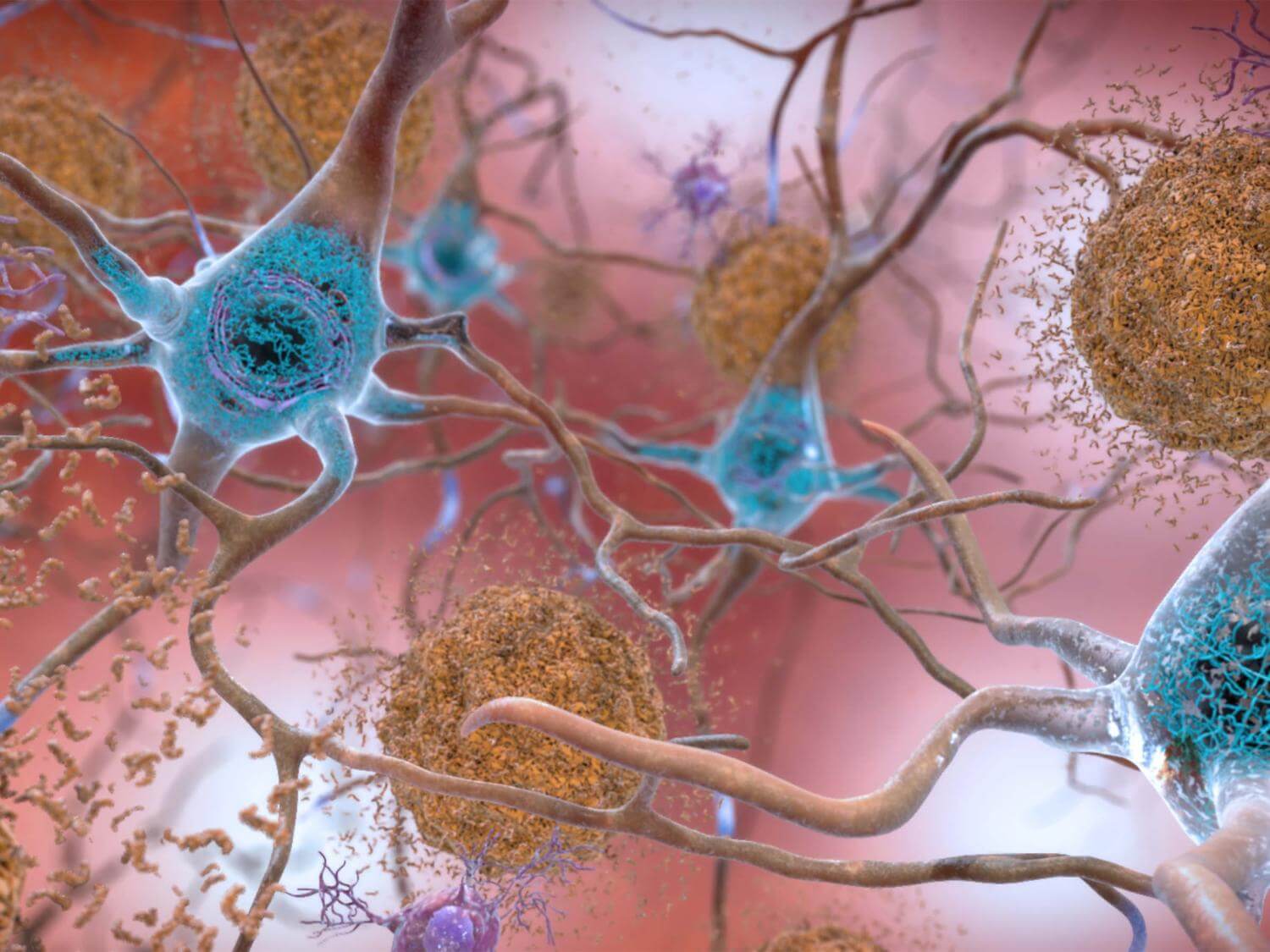
However, recent findings propose that disruptions to other brain processes, such as increased brain inflammation, might be just as crucial as amyloid buildup in triggering the cascade of neuronal death that causes rapid cognitive decline. The Pittsburgh team’s research has now demonstrated that cognitive impairment can be anticipated using a blood test.
Prof. Pascoal clarified that astrocytes are specialized cells abundant in brain tissue. They supply neuronal cells with nutrients and oxygen and safeguard them against pathogens. Despite their supportive roles, their function in health and disease had been underestimated because they don’t conduct electricity and didn’t initially appear to play a direct role in neuron communication.
“Astrocytes coordinate brain amyloid and tau relationship like a conductor directing the orchestra,” says lead author of the study Bruna Bellaver, Ph.D., postdoctoral associate at Pitt. “This can be a game-changer to the field, since glial biomarkers in general are not considered in any main disease model.”
(Photo by Los Muertos Crew from Pexels)
You might also be interested in:
- Blood testing for one specific protein may detect Alzheimer’s 10 years before symptoms emerge
- Early onset high blood pressure can alter the brain, increase risk of Alzheimer’s disease
The team examined blood samples from participants in three separate studies of cognitively unimpaired elderly people, testing for astrocyte reactivity markers and the presence of pathological tau. Only those who tested positive for both amyloid and astrocyte reactivity showed signs of progressively developing tau pathology, indicating a predisposition to Alzheimer’s disease symptoms.
Dr. Bellaver claims that these findings could have direct implications for future clinical trials for Alzheimer’s drug candidates. As trials aim to stop disease progression earlier, they are targeting ever earlier stages of pre-symptomatic disease, making accurate early diagnosis of Alzheimer’s risk crucial for success.
She emphasized that including astrocyte reactivity markers in tests would enhance the selection of patients likely to progress to Alzheimer’s later stages. This would allow more accurate patient selection for therapeutic interventions, benefiting those most likely to respond to treatment.
South West News Service writer Stephen Beech contributed to this report.

Are blood tests available to detect Alzheimer risk?