💡What To Know:
- In vitro gametogenesis transforms skin cells to become viable embryos.
- The process leads to fertilized embryos with chromosomes from both parents.
- IVG may help same-sex couples and women without viable eggs conceive.
PORTLAND, Ore. — A major breakthrough in fertility treatment may soon give same-sex couples the ability to have babies genetically related to both parents. This innovative technique addresses infertility by transforming a skin cell into an egg capable of producing viable embryos.
Researchers in Oregon believe this technology has the potential to benefit same-sex couples, women of advanced maternal age, and individuals unable to produce viable eggs, allowing them all to have healthy offspring with genetic contributions from both parents.
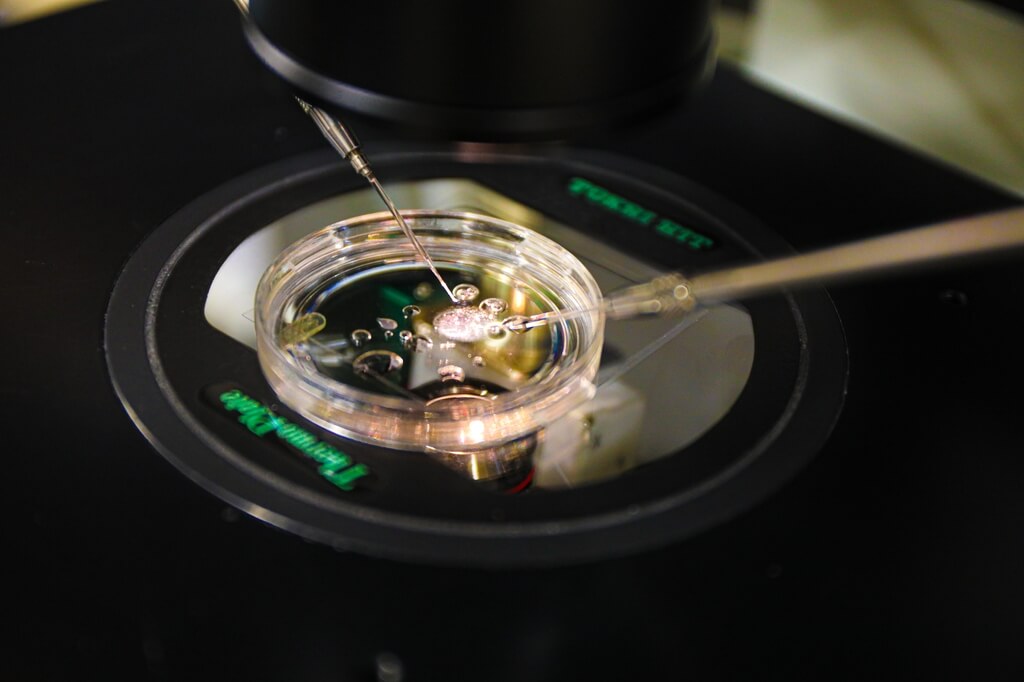
The team from the Oregon Health & Science University (OHSU) has demonstrated the process of in vitro gametogenesis (IVG) in mice. Their novel technique involves transferring the nucleus of a skin cell into a donated egg from which the original nucleus has been removed. The team then modifies the nucleus of the skin cell, halving its chromosome count to enable fertilization by a sperm cell and the creation of a viable embryo.
“The goal is to produce eggs for patients who don’t have their own eggs,” says senior author Shoukhrat Mitalipov, Ph.D., director of the OHSU Center for Embryonic Cell and Gene Therapy, and professor of obstetrics and gynecology, and molecular and cellular biosciences, in the OHSU School of Medicine, in a university release.
The study, featured in the journal Science Advances, opens new possibilities for older women and individuals unable to produce eggs due to cancer or other medical conditions to have babies. Additionally, it introduces the potential for men in same-sex relationships to have children genetically related to both partners.
Rather than trying to convert induced pluripotent stem cells (iPSCs) into sperm or egg cells, researchers at the OHSU refined a technique called somatic nuclear transfer. This method involves transplanting the nucleus of a skin cell into a donor egg from which the original nucleus has been removed. This approach was notably used in 1996 by scientists in Scotland to clone Dolly the sheep using cells from a single parent.
However, the OHSU team achieved results that allow for embryos with chromosomes from both parents. The procedure begins with transplanting a mouse skin cell nucleus into an egg that has had its nucleus removed. Once inside the donor egg, the skin cell nucleus sheds half of its chromosomes, a process encouraged by the egg’s cytoplasm. This step mirrors meiosis, the cell division process that generates mature sperm or egg cells, resulting in a haploid egg containing a single chromosome set.
From there, the researchers fertilize this egg with sperm through in vitro fertilization, creating a diploid embryo with two chromosome sets. This leads to the development of healthy offspring with genetic contributions from both parents.

Building on their initial proof of concept published in January 2022, the research team’s latest study advances their findings by meticulously sequencing the chromosomes. They observed that each time a skin cell nucleus was implanted into a donor egg, it successfully divided its chromosomes, with perfect segregation occurring in rare instances. This resulted in a harmonious match between the egg and sperm chromosomes, underscoring the technique’s potential for reproductive medicine.
“This publication basically shows how we achieved haploidy,” Mitalipov says. “In the next phase of this research, we will determine how we enhance that pairing so each chromosome pair separates correctly.”
While labs worldwide are using IVG techniques involving a time-intensive process of reprogramming skin cells to become iPSCs and then differentiating them to become egg or sperm cells, the OHSU team explains that they are leaving that step out of the process.
“We’re skipping that whole step of cell reprogramming,” adds co-author Paula Amato, M.D., professor of obstetrics and gynecology in the OHSU School of Medicine. “The advantage of our technique is that it avoids the long culture time it takes to reprogram the cell. Over several months, a lot of harmful genetic and epigenetic changes can happen.”
Although researchers are also studying the technique in human eggs and early embryos, Dr. Amato adds that it will likely be years before the technique is ready for clinical use.
“This gives us a lot of insight,” Dr. Amato concludes. “But there is still a lot of work that needs to be done to understand how these chromosomes pair and how they faithfully divide to actually reproduce what happens in nature.”
