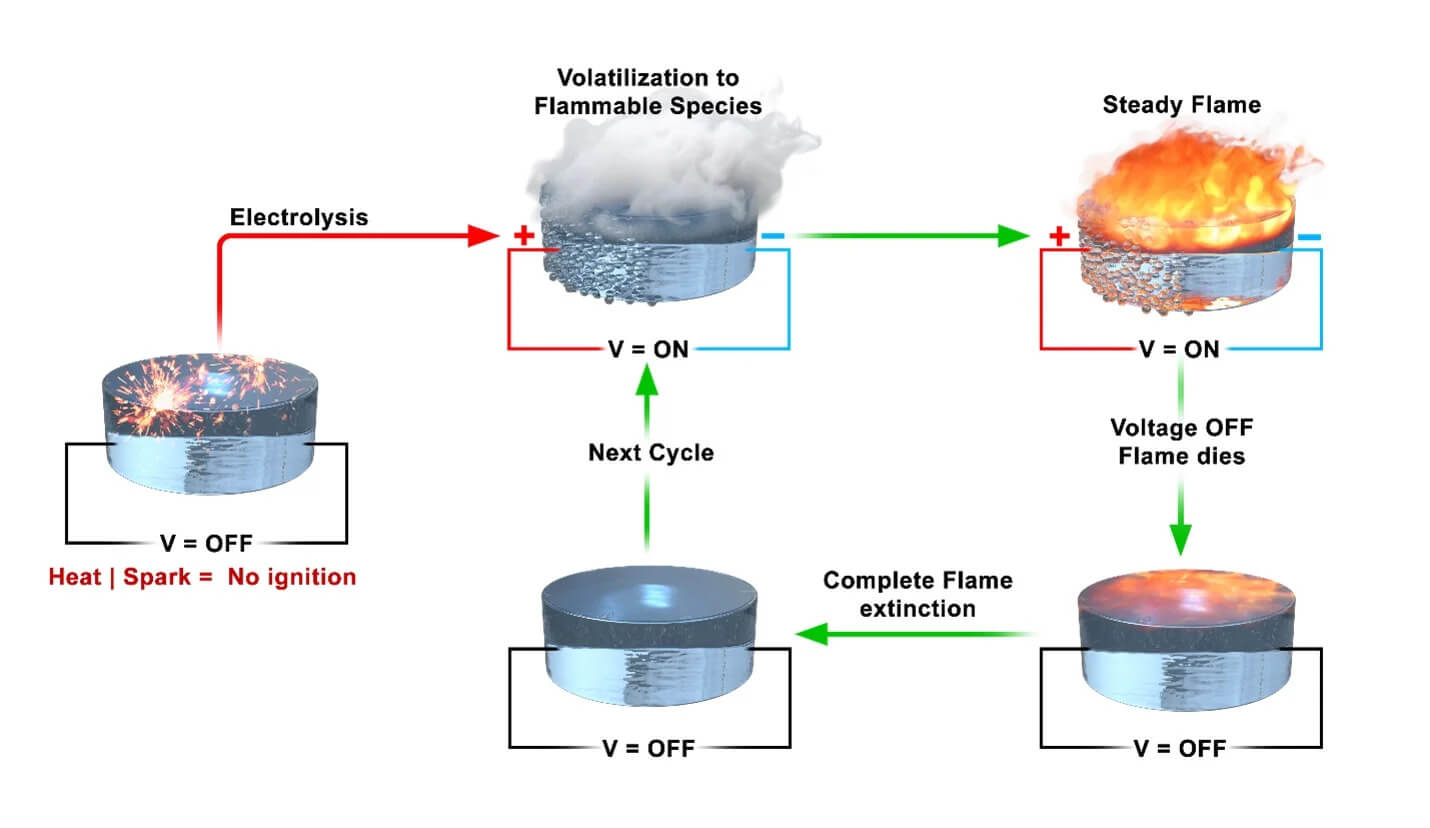
RIVERSIDE, Calif. — Can fuel be fireproof? It can now. Chemical engineers from the University of California-Riverside have designed a unique fuel that only ignites when applying an electrical current. The primary advantage of this innovative fuel is its safety — it doesn’t react to open flames, making it immune to accidental fires during storage or transportation.
“The fuel we’re normally using is not very safe. It evaporates and could ignite, and it’s difficult to stop that,” says study co-author Yujie Wang, a UCR chemical engineering doctoral student, in a university release. “It is much easier to control the flammability of our fuel and stop it from burning when we remove voltage.”
The study authors explain the creation process for this fuel. Researchers also filed a patent detailing further technical specifics.
What makes gasoline so flammable?
When a fuel burns, it’s not the liquid but the volatile fuel molecules above it that catch fire upon mixing with oxygen and flame.
“If you throw a match into a pool of gasoline on the ground, it’s the vapor of the gas that’s burning. You can smell that vapor and you instantly know it’s volatile,” says study first author Prithwish Biswas, a UCR chemical engineering doctoral student. “If you can control the vapor, you can control whether the fuel burns.”
At the heart of this innovation is an ionic liquid, which is essentially a liquified salt.
“It is similar to the salt we use to flavor food, which is sodium chloride,” notes Wang. “The one we used for this project has a lower melting point than table salt, low vapor pressure, and is organic.”

To test their creation, scientists used a regular cigarette lighter on the liquid. It didn’t ignite. However, when they applied an electric current and then used the lighter, the fuel ignited, but it stopped burning once the current was removed.
“Once we shut off the current, the flame was gone, and we were able to repeat that process over and over again — applying voltage, seeing smoke, lighting the smoke so it burned, then turning it off,” explains Wang. “We were excited to find a system we could start and stop very quickly.”
The more voltage applied, the larger and more energetic the flame became, which could offer precise control in engine systems.
“You can measure the combustion in this way, and cutting the voltage works like a dead man switch — a safety feature that automatically shuts down a machine if the operator becomes incapacitated,” says study corresponding author Michael Zachariah, a distinguished professor of chemical engineering at UCR.
In theory, this ionic liquid fuel could be utilized in any vehicle. Still, there are challenges to address before it can be brought to market, including engine compatibility and efficiency evaluations. One intriguing aspect is the fuel’s ability to mix with conventional fuels while retaining its non-flammable properties, though the exact mix ratio remains a subject of further research.
While excited about the safety benefits of their invention, Zachariah pointed out potential hurdles in commercializing the product, primarily due to its current higher cost.
“This would definitely be more expensive than the way they currently manufacture fuels. These compounds are not normally produced in bulk, but if they were, the cost would go down,” says Zachariah. “How competitive would it be? I don’t know. But if safety is important, that’s a major aspect of this. You make something safe, then there is a benefit that goes beyond the bottom line.”
The study is published in the Journal of the American Chemical Society.
You might also be interested in:
- Best Cars For Gas Mileage In 2023: Top 5 Fuel-Efficient Vehicles, According To Experts
- Best Electric Cars: Top 5 EV Models Recommended By Experts
- Star Trek-style ‘replicator’ harvests water from thin air to make clean fuel

Lea la versión en español en EstudioRevela.com: Ingenieros químicos inventan un combustible revolucionario que no se incendiará.

Obviously the first question the current zeitgeist will ask is – is it environmentally friendly? What are the products of its combustion?