🔑 Key Findings:
- Leaks in the cells’ mitochondria may be causing autoimmune conditions.
- DNA escaping this cell “battery” can trigger harmful inflammation.
- Scientists believe that targeting this problem can reduce disease and slow aging.
CHARLOTTESVILLE, Va. — There could soon be hope for people suffering from inflammatory diseases. Scientists from the University of Virginia Health System have unraveled a cellular mystery that may revolutionize the treatment of lupus and rheumatoid arthritis. The key lies within our cells’ mitochondria, often referred to as the powerhouses of the cell, which can become “leaky” and trigger harmful inflammation.
This discovery opens the door to developing therapies that could not only tackle autoimmune diseases but also enhance our virus-fighting capabilities and potentially slow down the aging process.
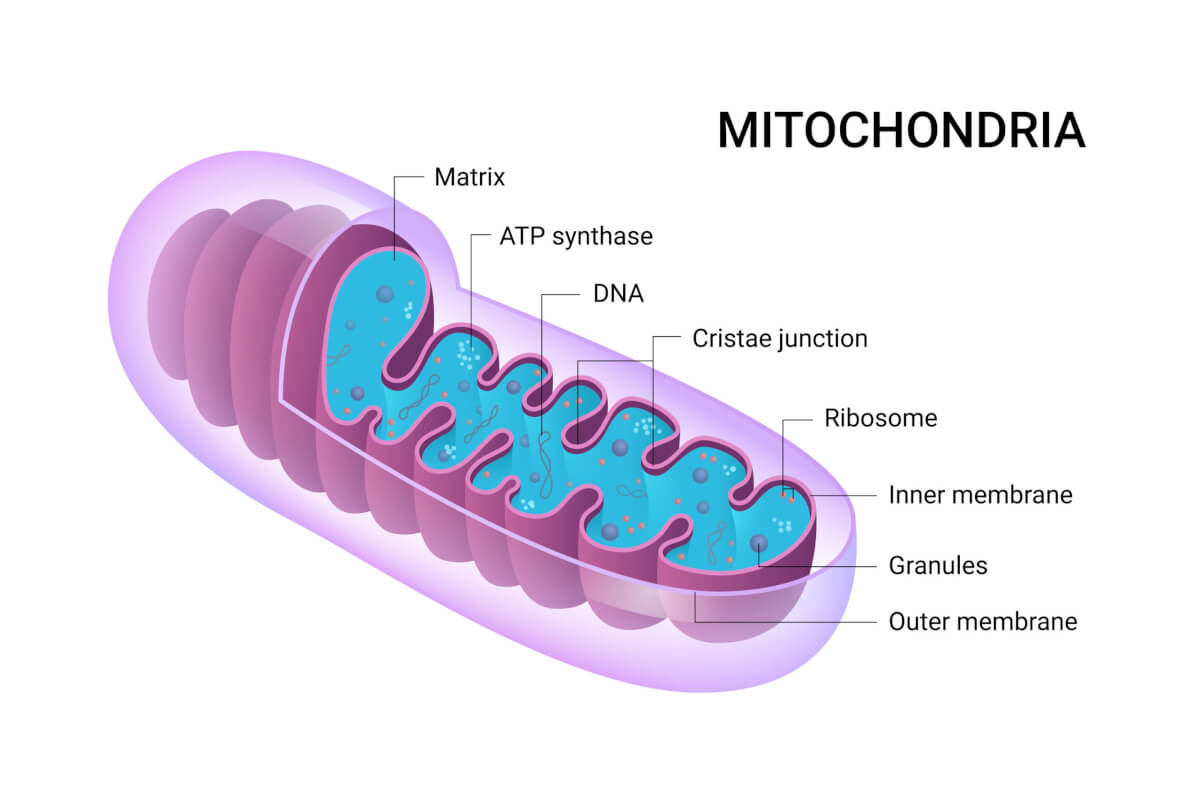
Mitochondria are unique in that they contain their own genetic material, known as mitochondrial DNA (mtDNA), which operates separately from the cell’s main DNA. It’s been known that when mtDNA escapes from the mitochondria, it can provoke inflammation. However, the precise mechanics behind this leakage and the subsequent inflammatory response were not fully understood until now.
Researchers have identified a malfunction in the replication of mtDNA as the culprit. This glitch leads to the build-up of protein clusters known as nucleoids within the mitochondria. In an attempt to rectify this issue, the cell tries to expel these excess nucleoids to its “trash bins” or endosomes. When these endosomes become overwhelmed, though, they spill the mtDNA into the cell, akin to an overflowing trash can.
This spillage prompts the cell to recognize the nucleoids as foreign invaders, similar to a virus, and mount an immune response that results in inflammation.
“When mitochondria don’t correctly replicate their genetic material, they try to eliminate it. However, if this is happening too often and the cell can’t dispose of all of it, it can cause inflammation, and too much inflammation can lead to disease, including autoimmune and chronic diseases,” says lead researcher Dr. Laura E. Newman, of the University of Virginia School of Medicine, in a media release. “Now that we are beginning to understand how this inflammation starts, we might be able to prevent this process, with the ultimate goal of limiting inflammation and treating disease.”
Researchers utilized advanced imaging and cell biology techniques to track the pathway of mtDNA from the mitochondria to the cell’s interior.
“We knew that mtDNA was escaping mitochondria, but how was still unclear,” notes Dr. Gerald Shadel, director of the San Diego-Nathan Shock Center of Excellence in the Basic Biology of Aging at the Salk Institute. “Using imaging and cell biology approaches, we’re able to trace the steps of the pathway for moving mtDNA out of the mitochondria, which we can now try to target with therapeutic interventions to hopefully prevent the resulting inflammation.”
This discovery not only sheds light on the process but also identifies potential targets for therapeutic intervention to halt the harmful inflammation.
“We had a huge breakthrough when we saw that mtDNA was inside of a mysterious membrane structure once it left mitochondria. After assembling all of the puzzle pieces, we realized that structure was an endosome,” adds Dr. Newman. “That discovery eventually led us to the realization that the mtDNA was being disposed of and, in the process, some of it was leaking out.”
The implications of this research are vast, with potential impacts on the treatment of diseases, our understanding of the immune response to viral infections, and the aging process.
“Using our cutting-edge imaging tools for probing mitochondria dynamics and mtDNA release, we have discovered an entirely novel release mechanism for mtDNA,” explains researcher Dr. Uri Manor, former director of the Waitt Advanced Biophotonics Core at Salk and current assistant professor at the University of California-San Diego. “There are so many follow-up questions we cannot wait to ask, like how other interactions between organelles control innate immune pathways, how different cell types release mtDNA, and how we can target this new pathway to reduce inflammation during disease and aging.”
As the research team continues to explore this novel mechanism, there is hope that their findings will lead to better treatments for inflammatory diseases and a deeper understanding of how our bodies respond to cellular stress and infections.
The study is published in the journal Nature Cell Biology.
Why don’t these payloads end up in lysosomes where they can be degraded. Endosomes generally fuse with lysosomes.