OSAKA, Japan — A new “fountain of youth” may be hiding inside our own cells, a new study explains. Scientists at Osaka University in Japan have identified a protein connected to how cells maintain their health, potentially opening doors to new anti-aging treatments and remedies for age-related diseases. The protein, HKDC1, plays a crucial role in preserving two essential components of a cell — the mitochondria and lysosomes.
Mitochondria are often described as the powerhouses of the cell, providing energy for cellular functions. Lysosomes, on the other hand, act as the cell’s waste disposal system. The proper functioning of these organelles is vital for the health of the cell, and their damage has been linked to aging, cellular senescence (the process by which cells stop dividing), and various diseases.
Previous studies hinted that a protein named TFEB was involved in maintaining the function of both mitochondria and lysosomes. However, the exact mechanisms were unknown. The Osaka University team discovered that TFEB directly targets the gene encoding HKDC1. Under stress conditions affecting mitochondria or lysosomes, HKDC1 is upregulated — meaning its production is increased.
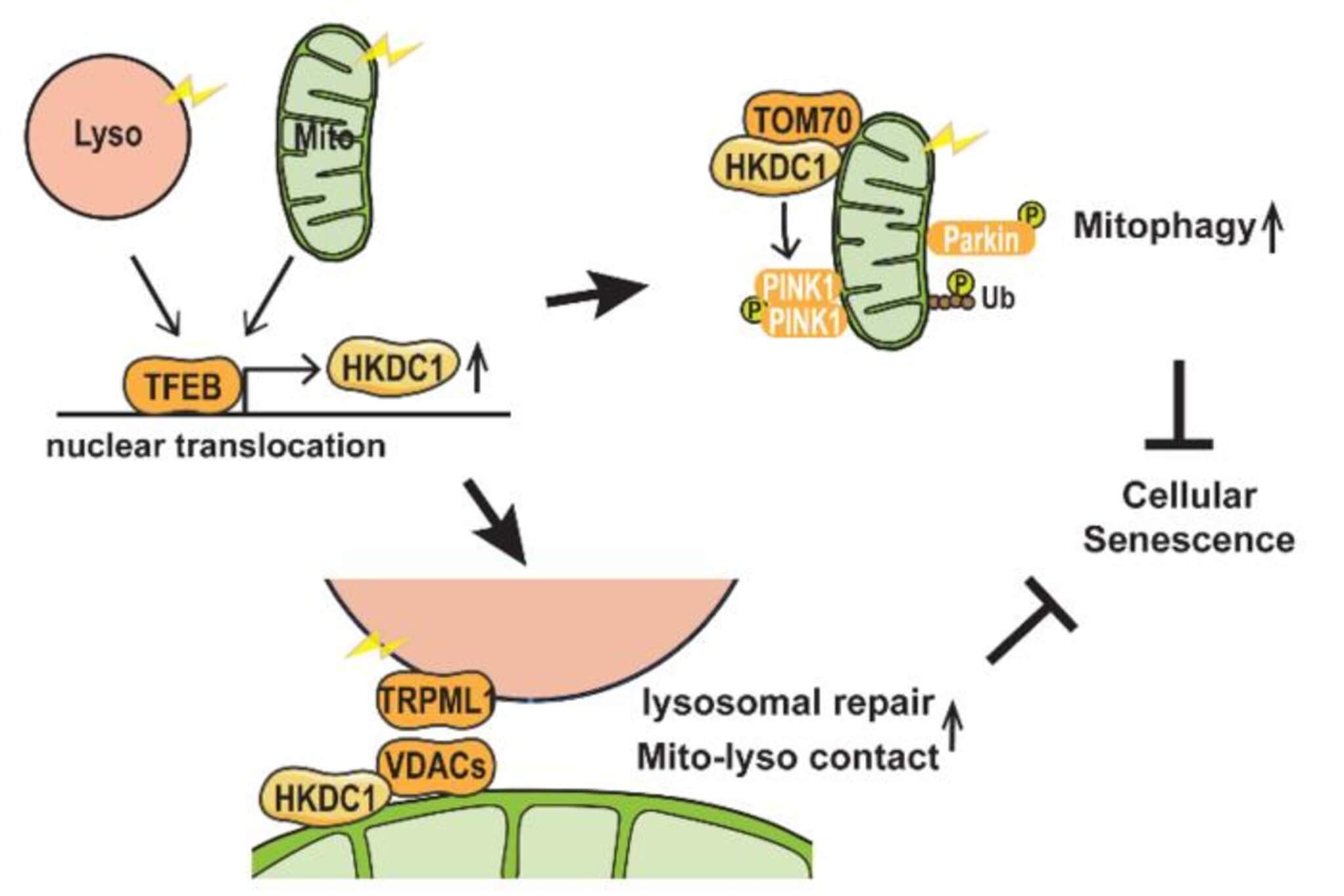
One of the key processes for protecting mitochondria is called mitophagy. It involves the selective removal of damaged mitochondria to prevent harm to the cell. This process usually relies on proteins called PINK1 and Parkin.
“We observed that HKDC1 co-localizes with a protein called TOM20, which is located in the outer membrane of the mitochondria, and through our experiments, we found that HKDC1, and its interaction with TOM20, are critical for PINK1/Parkin-dependent mitophagy,” says study lead author Mengying Cui, of Osaka University, in a media release.
In simpler terms, HKDC1, guided by TFEB, assists in removing damaged mitochondria. However, HKDC1’s role isn’t limited to mitochondria. It also plays a vital part in lysosomal repair. The team found that reducing HKDC1 in cells interfered with the repair process of lysosomes.
“HKDC1 is localized to the mitochondria, right? Well, this turns out to also be critical for the process of lysosomal repair,” explains senior author Shuhei Nakamura. “You see, lysosomes and mitochondria contact each other via proteins called VDACs. Specifically, HKDC1 is responsible for interacting with the VDACs; this protein is essential for mitochondria–lysosome contact, and thus, lysosomal repair.”
The dual functionality of HKDC1 in both lysosomes and mitochondria is a critical factor in preventing cellular senescence. By maintaining the stability of these organelles, HKDC1 helps keep cells healthy and functioning properly. Given the link between organelle dysfunction and aging, as well as age-related diseases, this discovery presents a path to new potential therapeutics for treating such conditions.
The study is published in the journal Proceedings of the National Academy of Sciences.
You might also be interested in:
- Best Anti-Aging Serums: Top 7 Brands Most Recommended By Beauty Experts
- Taurine supplements could be the secret to unlocking the fountain of youth
- Aging gracefully? Poll finds young adults more afraid of getting older than seniors

