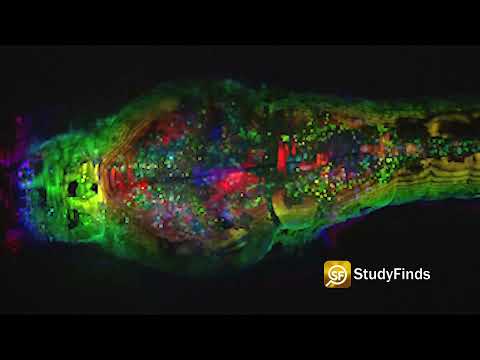
ROCHESTER, N.Y. — Researchers may be a step closer to understanding neurodegenerative diseases, such as Huntington’s disease and schizophrenia. For the first time, scientists from the University of Rochester have created highly detailed 3D images of the synapse, the critical junction where neurons communicate through chemical signals. These intricate models mark a remarkable technical achievement, enabling researchers to examine the various cells that converge at individual synapses with an unprecedented level of detail.
“It is one thing to understand the structure of the synapse from the literature, but it is another to see the precise geometry of interactions between individual cells with your own eyes,” states Abdellatif Benraiss, Ph.D., a research associate professor in the Center for Translational Neuromedicine and co-author of the study, in a university release. “The ability to measure these extremely small environments is a young field and holds the potential to advance our understanding of a number of neurodegenerative and neuropsychiatric diseases in which synaptic function is disturbed.”
The researchers utilized this novel technique to compare the brains of healthy mice with those carrying the mutant gene responsible for Huntington’s disease. Previous research conducted by Goldman’s team has indicated that dysfunctional astrocytes, a type of support cell in the brain, play a crucial role in the disease. Astrocytes help maintain the appropriate chemical environment at the synapse.
Specifically, the study focused on synapses involving medium spiny motor neurons, whose progressive loss characterizes Huntington’s disease. To identify these synapses among the complex network of cells, the researchers employed fluorescent tags assigned by viruses to the axons, motor neurons, and astrocytes. After imaging the areas of interest using multiphoton microscopy, the team used infrared branding to create reference points in the brain tissue for later relocation.

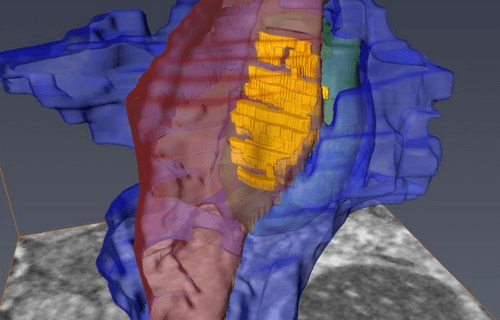
The brain tissue was then examined using a serial block-face scanning electron microscope at the University of Copenhagen, designed to study the brain’s smallest structures. This advanced device, utilizing a diamond knife, sequentially removed and imaged ultrathin slices of brain tissue, ultimately generating 3D models at the nanometer scale that showcased the labeled cells and their interactions at the synapse.
Analysis of the brain tissue from healthy mice revealed that astrocytes fully enveloped and tightly bonded with the disk-shaped synapse. In contrast, the astrocytes in Huntington’s mice exhibited less effective investment or sequestration of the synapse, resulting in significant gaps. This structural flaw allows potassium and glutamate, crucial chemicals for cell communication, to leak from the synapse, potentially disrupting normal functioning.
Astrocyte dysfunction has been associated with other conditions like schizophrenia, amyotrophic lateral sclerosis, and frontotemporal dementias. The researchers believe that this technique could greatly enhance the understanding of the precise structural basis for these diseases. Importantly, it could be employed to evaluate the effectiveness of cell replacement strategies, which involve replacing diseased glial cells with healthy ones, as potential treatments for these conditions.
The study is published in the journal PNAS.