NEW YORK — A highly used drug treatment could potentially cause a serious side-effect among breast cancer patients. New findings have highlighted an increased rate of high blood sugar, technically known as hyperglycemia, among breast cancer patients undergoing treatment with the oral drug alpelisib — better known under the brand name Piqray.
Alpelisib is designed to target a protein called phosphoinositide 3-kinase (PI3K), responsible for cell growth. PI3K, when mutated, can play a role in cancer progression. Recognizing its potential, the U.S. Food and Drug Administration (FDA) gave the green light in 2019 for alpelisib’s use, in tandem with fulvestrant (an estrogen receptor blocker), for specific instances of metastatic breast cancer associated with mutations in the PI3K gene.
However, there’s a hitch. Targeting PI3K with alpelisib can usher in the unwanted side-effect of hyperglycemia. When acute, hyperglycemia can cause severe dehydration or kidney damage, occasionally necessitating hospital admission.
Memorial Sloan Kettering Cancer Center scientists explored the occurrence, potential risks, and treatment strategies for hyperglycemia linked with alpelisib. Their study covered patients with metastatic breast cancer treated either conventionally or as part of a clinical trial.
Their data was telling. A staggering 80.3 percent of the 147 patients on standard care with alpelisib experienced hyperglycemia. Of these, 40.2 percent faced severe cases. Meanwhile, out of the 100 clinical trial participants, 34 percent encountered any grade of hyperglycemia, with 13 percent dealing with severe forms. It took an average of just 16 days post-alpelisib treatment for hyperglycemia to appear among patients. One notable risk factor was a high initial level of hemoglobin A1c, which signals elevated blood sugar levels akin to pre-diabetes or diabetes conditions.
For those who grappled with elevated blood sugar, 66.4 percent received remedial treatment, with the diabetes medication metformin being a common choice.
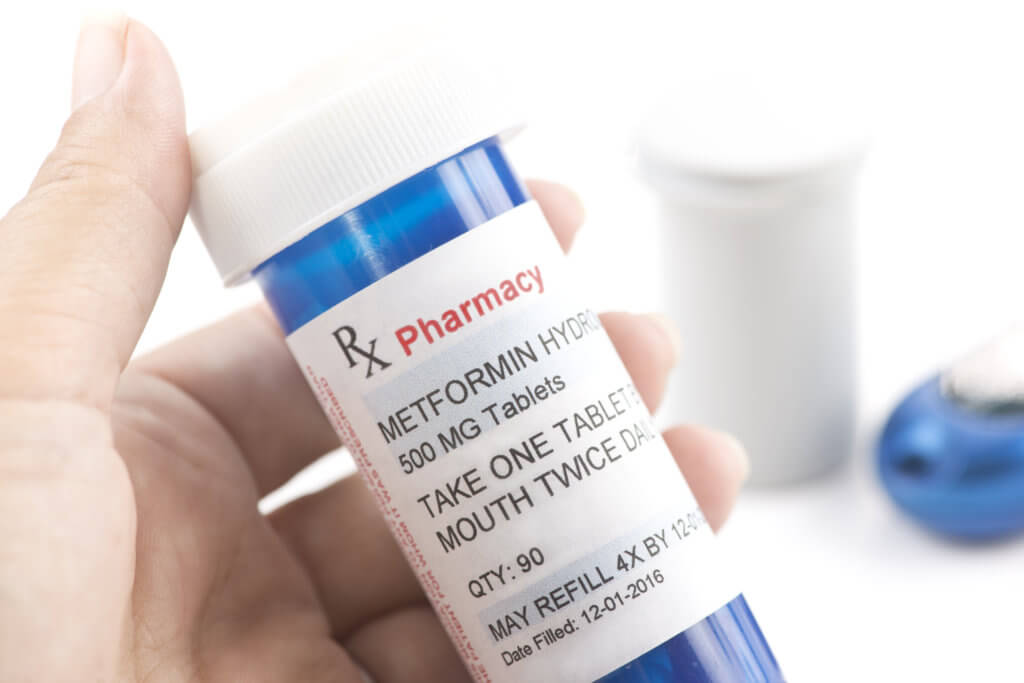
“If a patient is identified to have a PI3KCA mutation and thus eligible for treatment with alpelisib, we should be checking hemoglobin A1c level and partnering with the patient’s primary care physician and/or endocrinologist to optimize their blood sugar levels,” says study author Dr. Sherry Shen of Memorial Sloan Kettering Cancer Center in a media release.
“This needs to be done months before initiating alpelisib, because once alpelisib is started, hyperglycemia usually develops within the first two weeks of treatment. Being pre-emptive about improving glycemic status and treating prediabetes/diabetes will hopefully lower the patient’s risk of developing hyperglycemia and thus, lower their risk of needing to discontinue a drug that could be effective for their cancer.”
Dr. Neil M. Iyengar, the study’s senior author, adds that managing a patient’s blood sugar frequently requires lifestyle changes and, at times, certain medications.
“Improving metabolic risk factors through lifestyle interventions may also improve dose delivery of alpelisib, and ongoing clinical trials by our group and other groups are testing whether metabolic interventions such as the ketogenic diet or newer medications used to treat diabetes could also improve the treatment efficacy of cancer therapies that target the PI3K pathway,” notes Dr. Iyengar.
The study is published in the journal CANCER.

Wait till AI takes over and we all become Doc Oc!