GUANGDONG, China — In a groundbreaking experiment, scientists have successfully grown hybrid kidneys consisting of both human and pig cells inside pigs. This research aims to pave the way for generating other human organs in pigs, such as hearts and pancreas, with the ultimate objective of optimizing the technology for human organ transplantation.
This advancement in kidney development marks the first time that researchers have successfully grown a “humanized” solid organ inside another species. Led by a team from the Guangzhou Institutes of Biomedicine and Health, researchers focused on kidneys because they are among the first organs to develop and are the most frequently transplanted organs.
“Rat organs have been produced in mice, and mouse organs have been produced in rats, but previous attempts to grow human organs in pigs have not succeeded,” says senior author Professor Lai Liangxue in a media release. “Our approach improves the integration of human cells into recipient tissues and allows us to grow human organs in pigs.”
The researchers explain that incorporating human stem cells into pig embryos has been challenging due to competition from pig cells and the different physiological requirements between pig and human cells.
“We have been working on mechanisms to overcome the extremely low efficiency in interspecies chimera. We identified a couple of critical factors that enhance the formation of interspecies chimera by facilitating cell competition,” adds another senior author, Professor Guangjin Pan.
The team’s approach relies on three key techniques:
1. Creating a niche within the pig embryo, using CRISPR technology, to eliminate competition from pig cells. This was done by genetically engineering the pig embryo to lack two genes essential for kidney development.
2. Modifying human pluripotent stem cells—cells with the potential to become any cell type — to enhance their integration and survival. This was achieved by temporarily inhibiting apoptosis, or programmed cell death.
3. Culturing these engineered stem cells in a special medium to convert them into “naïve” cells, resembling early human embryonic cells.
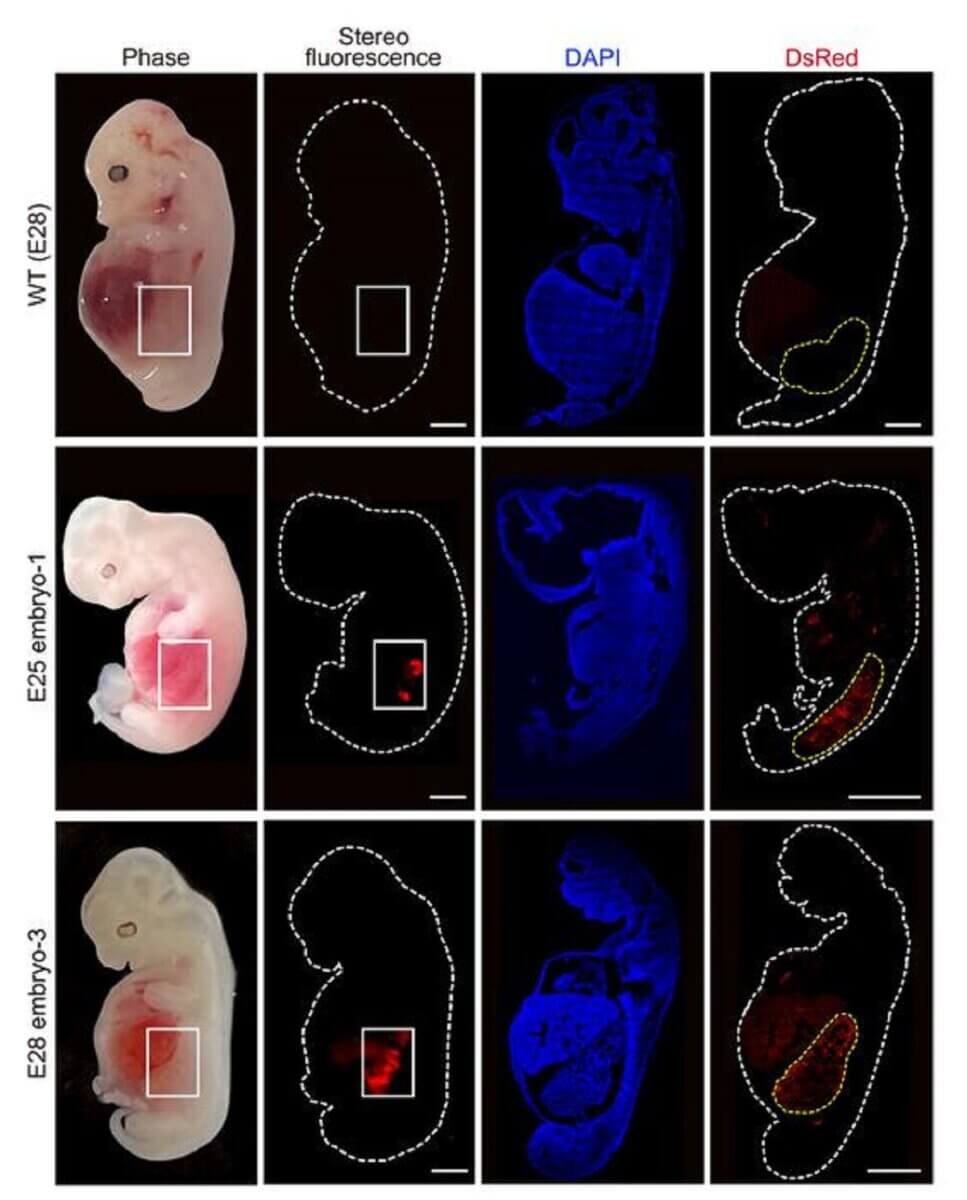
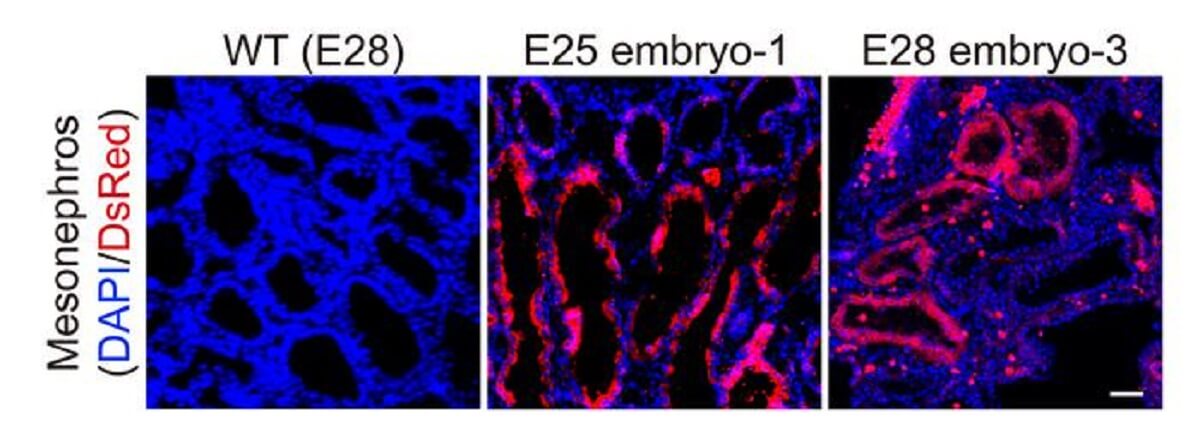
The research team transferred a total of 1,820 embryos into 13 surrogate pig mothers. After 25 to 28 days, they terminated the pregnancies to examine the embryos. They found that the kidneys had a normal structure and were composed of 50 to 60 percent human cells at their stage of development.
The team explains that human cells were largely confined to the kidneys, thereby reducing ethical concerns about the potential presence of human cells in neural or germline tissues.
“Because organs are not composed of just one cell lineage, in order to have an organ where everything comes from the human, we would probably need to engineer the pigs in a much more complex way and that also brings some additional challenge,” says Dr. Miguel Esteban, a senior author of the study. “You can trace the human cells you’re injecting and manipulate them so that you can study diseases and how cell lineages are formed.”
The research is published in the journal Cell Stem Cell.
South West News Service writer Stephen Beech contributed to this report.

When will this be available to joe public? Do they need people as test subjects