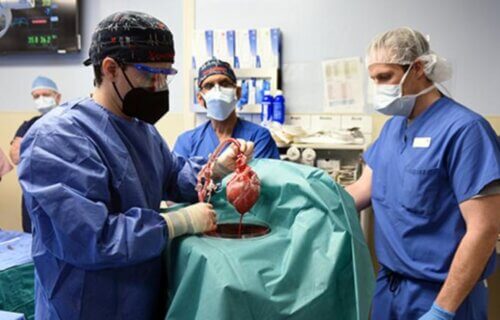
BALTIMORE — In 2022, physician-scientists from the University of Maryland School of Medicine (UMSOM) conducted the world’s first pig-to-human heart transplant, an important breakthrough in the field of medicine. The patient survived for almost two months before experiencing a rapid decline that led to his death. Now, the scientists are sharing what they learned from their work and how it’ll prepare them for future transplants.
“Our paper provides crucial insight into how a multitude of factors likely played a role in the functional decline of the transplanted heart,” says study lead author Muhammad M. Mohiuddin, MD, Professor of Surgery and Scientific/Program Director of the Cardiac Xenotransplantation Program at UMSOM, in a media release. “Our goal is to continue moving this field forward as we prepare for clinical trials of xenotransplants involving pig organs.”
The patient was 57-year-old David Bennett, Sr., a man with end-stage heart failure that did not qualify for a traditional heart transplant. The U.S. Food and Drug Administration (FDA) authorized the transplant under their expanded access (compassionate use) provision. Initially after his treatment, he didn’t show signs of acute rejection for almost seven weeks post-surgery. His decline started after a sudden onset of heart failure nearly two months later. Since then, the transplant team has been studying the physiological mechanisms responsible for the heart failure extensively in order to help the odds of future patients.
“We were determined to shed light on what led to the heart transplant dysfunction in Mr. Bennett, who performed a heroic act by volunteering to be the first in the world,” says study co-author Bartley Griffith, MD, Professor of Surgery and The Thomas E. and Alice Marie Hales Distinguished Professor in Transplantation at UMSOM. “We want our next patient to not only survive longer with a xenotransplant but to return to normal life and thrive for months or even years.”
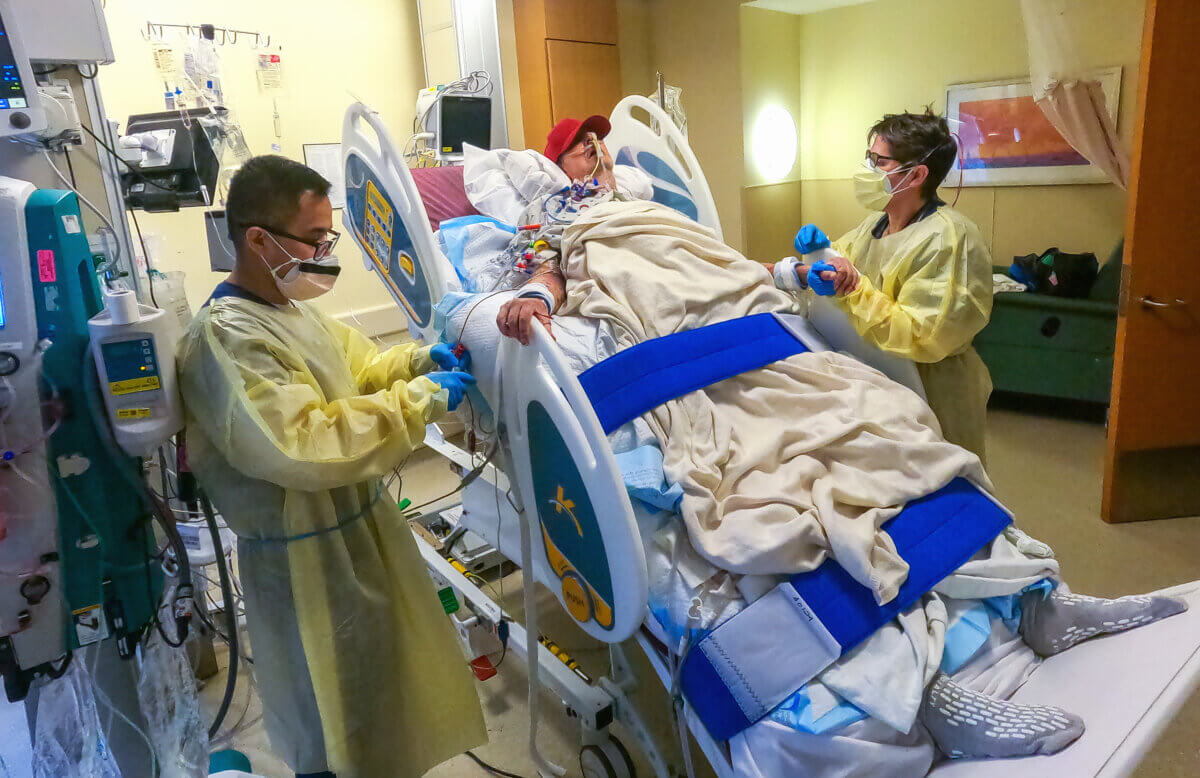
The team mapped out the sequence of events that led to the sudden heart failure using the limited tissues they could get from the patient. They found that the heart was functioning well until day 47 post-surgery. This confirmed that Bennett didn’t experience any signs of acute rejection until several weeks post-op. It’s likely that there are different overlapping factors that led to the eventual decline, such as him being immunocompromised and in poor health before the transplant.
As a result, implementing an effective anti-rejection routine used in preclinical studies for this type of transplantation was limited. Generally with transplants, recipients need to be on anti-rejection medications that impair the immune system for the rest of their lives in order to ensure the body doesn’t reject the transplanted part. The researchers think the patient was even more vulnerable rejection due to the antibodies produced by the immune system. They also found indirect evidence of antibody-mediated rejection based on histology, immunohistochemical staining and single cell RNA analysis.
Further, the team revealed that the use of an intravenous immunoglobulin, IVIG, a drug with antibodies, may also have contributed to heart muscle cell damage. The patient received the drug twice during the second month after the transplant to prevent infection, but this may also have triggered an immune response. They found evidence of immunoglobulin antibodies targeting the pig heart.
Finally, their study looked at the presence of a latent virus within the heart called porcine cytomegalovirus (PCMV), which may have contributed to things. Activation of the virus may have happened after the patient’s anti-viral treatment regimen was reduced to address other health problems he experienced. This could’ve subsequently caused an inflammatory, cell-damaging response. However, the team notes that there wasn’t a sign of infection in the patient nor one showing that the virus spread beyond the heart. For future transplants, better PCMV testing protocols have been developed for sensitive detection and exclusion of latent viruses.
“Valuable lessons can be learned from this groundbreaking surgery and the courageous first patient, Mr. Bennett, that will better inform us for future xenotransplants,” says UMSOM Dean Mark T. Gladwin, MD, Vice President for Medical Affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor. “In the future, our team of surgeon-scientists will utilize newly designed immune cell assays to monitor the patient more precisely in the days, weeks, and months following the xenotransplant. This will provide stricter control of the earliest signs of rejection and the promise of a truly lifesaving innovation.”