BEIJING, China — In the quest to tackle neurodegenerative diseases like Alzheimer’s and Parkinson’s, scientists have zeroed in on the accumulation of certain misfolded proteins called amyloids. This accumulation is thought to be a defining trait of these diseases. In essence, these proteins misbehave by clumping together, which can potentially damage brain cells. Now, scientists believe the cure may involve targeting them with high-frequency electromagnetic waves.
To understand this better, think of amyloid proteins like misfolded origami paper. When these proteins misfold, they bunch together in the wrong way. Like a chain reaction, once one protein misbehaves, others tend to follow, leading to a cascade of clumping, which isn’t good for the brain.
Researchers from Peking University may have found a way to regulate this clumping using a specific frequency, according to a media release. Their findings suggest that there’s a potential to slow or even prevent the onset of diseases like Alzheimer’s.
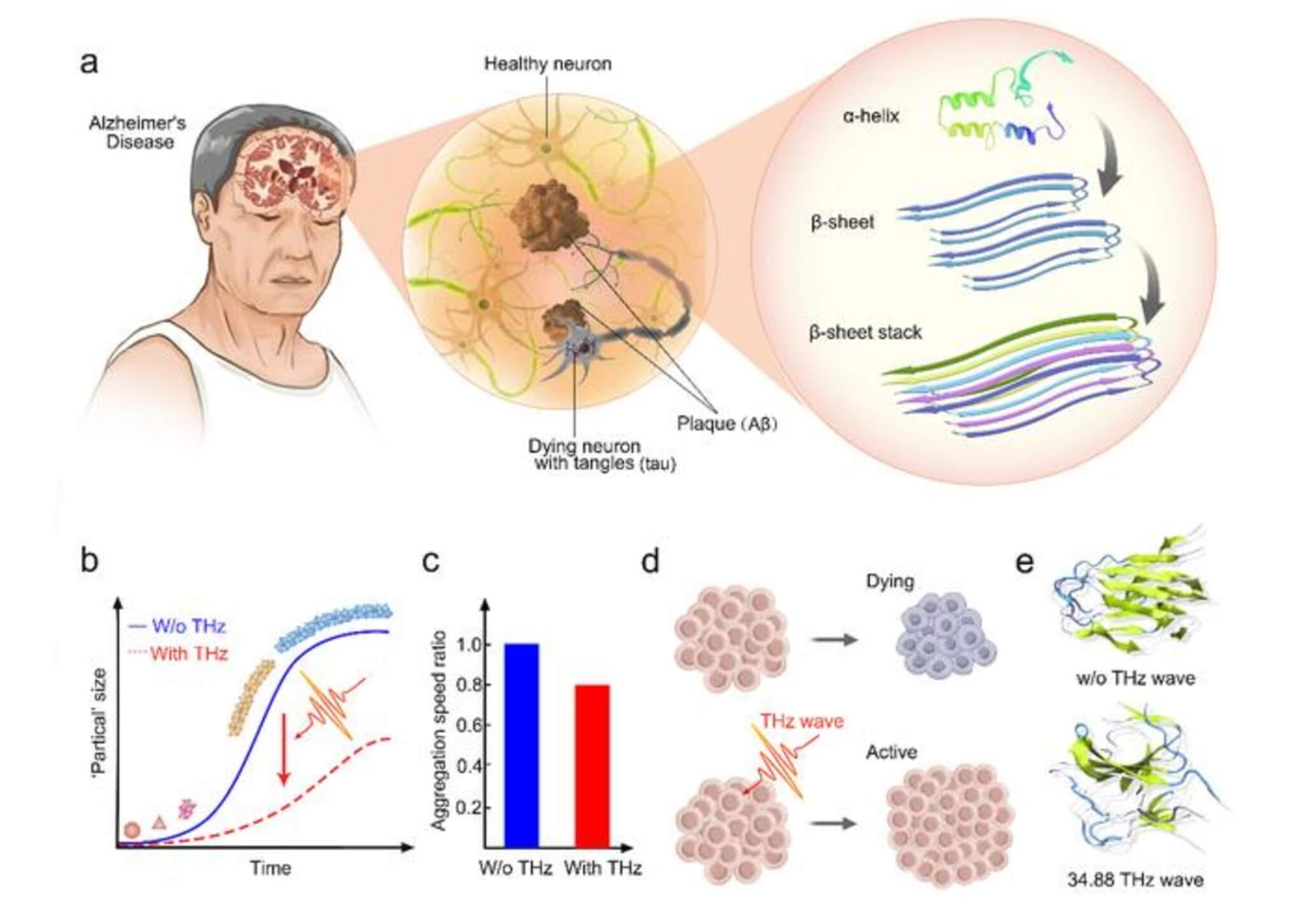
The research hinges on a concept proposed in 2018. It was believed that certain high-frequency waves, known as THz electromagnetic (EM) waves, could affect biological processes in our bodies. These waves resonate with molecules, especially changing the way they bond with each other. The scientists believed that if they could target these waves just right, they might prevent the unwanted clumping of amyloid proteins.
Earlier experiments hinted that certain light frequencies could break apart these troublesome protein accumulations. There was a catch — these frequencies also produced heat, which could be harmful. So, researchers searched for a safer, non-thermal way to achieve the same effect.
They used a specialized laser to shine a specific frequency of light onto samples of the amyloid protein, known as Aβ. They found that this treatment slowed down the protein’s clumping process. Tests revealed that this frequency was safe for cells and might even benefit their function.
The treatment didn’t just slow down the clumping — it changed the protein’s structure. It was like the misfolded origami paper was being corrected, preventing the chain reaction of clumping.
Researchers believe that targeting these proteins with THz waves might be a game-changer in the fight against Alzheimer’s. Although their research was based on Aβ, they speculate that similar tactics could be used against other proteins, like tau protein, opening doors to combined treatments.
The study is published in the journal Light Science & Applications.