CAMBRIDGE, United Kingdom — Get the textbook publishers on the phone. Long-standing educational models need a rewrite after a team of international researchers made a revolutionary discovery about the organization of water molecules in salt water. This finding is particularly significant for understanding climate-related and environmental processes.
At the heart of this discovery is the interaction between water molecules and air, a critical factor in many environmental reactions. For instance, the evaporation of ocean water is a key component in atmospheric chemistry and climate science. The way water molecules behave at the surface where they meet air has a direct impact on these processes.
“These types of interfaces occur everywhere on the planet, so studying them not only helps our fundamental understanding but can also lead to better devices and technologies,” says Mischa Bonn, professor and chair of the Molecular Spectroscopy Department of the Max Planck Institute, in a university release. “We are applying these same methods to study solid/liquid interfaces, which could have potential applications in batteries and energy storage.”
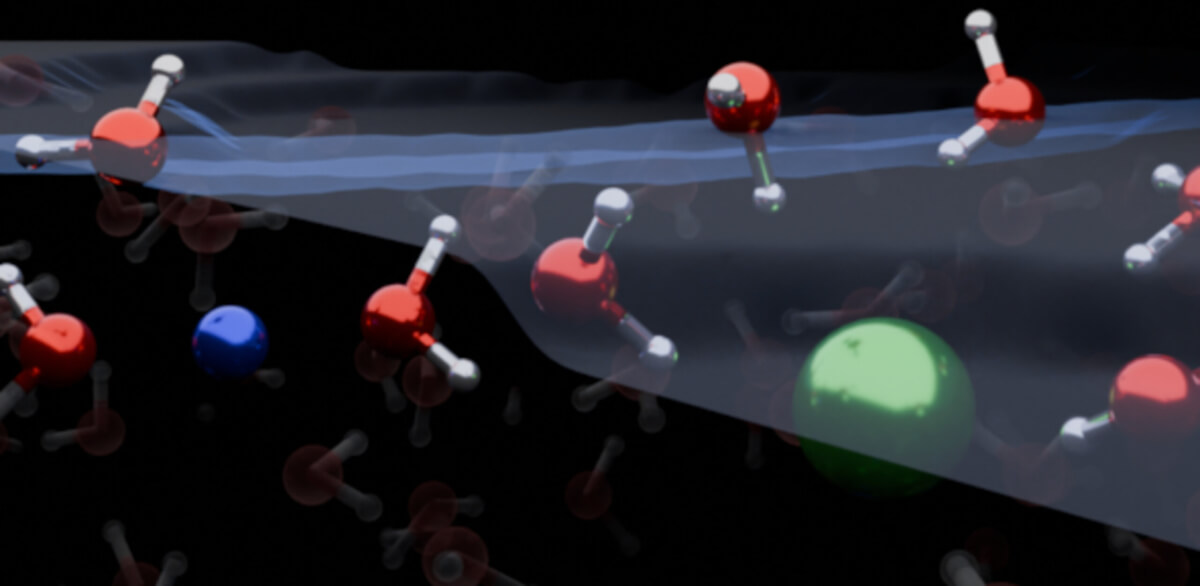
For years, the scientific community has debated the precise nature of these microscopic reactions at the air-water interface. The new study sheds light on this debate. Researchers from the Max Planck Institute for Polymer Research in Germany, along with colleagues from other institutions, found that the arrangement of ions and water molecules at the surface of salt water, or electrolyte solutions, is vastly different from previous beliefs.
The traditional method of studying these molecules, known as vibrational sum-frequency generation (VSFG), uses laser radiation to measure molecular vibrations at these critical interfaces. However, this technique has limitations; it doesn’t distinguish between positive and negative signals, leading to ambiguous interpretations.
To overcome these challenges, researchers employed an advanced version of VSFG, called heterodyne-detected (HD)-VSFG, and complemented this with sophisticated computer simulations. Their innovative approach revealed a surprising new picture of the saltwater surface.
“This paper shows that combining high-level HD-VSFG with simulations is an invaluable tool that will contribute to the molecular-level understanding of liquid interfaces,” notes study co-first author Dr. Kuo-Yang Chiang of the Max Planck Institute.
Contrary to what has been taught in textbooks, research demonstrated that both cations (positively charged ions) and anions (negatively charged ions) are scarce at the water/air interface. Instead of forming an electrical double layer and orienting water molecules in one direction, as previously thought, these ions influence water molecules to orient in multiple directions.
“Our work demonstrates that the surface of simple electrolyte solutions has a different ion distribution than previously thought and that the ion-enriched subsurface determines how the interface is organized: at the very top there are a few layers of pure water, then an ion-rich layer, then finally the bulk salt solution,” explains study co-first author Dr. Yair Litman, a theoretical chemist at the University of Cambridge.
The study is published in the journal Nature Chemistry.

Ahhh! Ok. Is it going to rain this week?