CHICAGO — The ALS Ice Bucket Challenge, which went viral several years ago, led to significant funding and the subsequent discovery of new genes tied to the disease. One of these genes, NEK1, is connected to approximately two percent of all cases of amyotrophic lateral sclerosis, positioning it as a major cause of the condition. Now, for the first time, Northwestern Medicine scientists have pinpointed exactly how this mutated gene leads to ALS, a debilitating neurodegenerative disease.
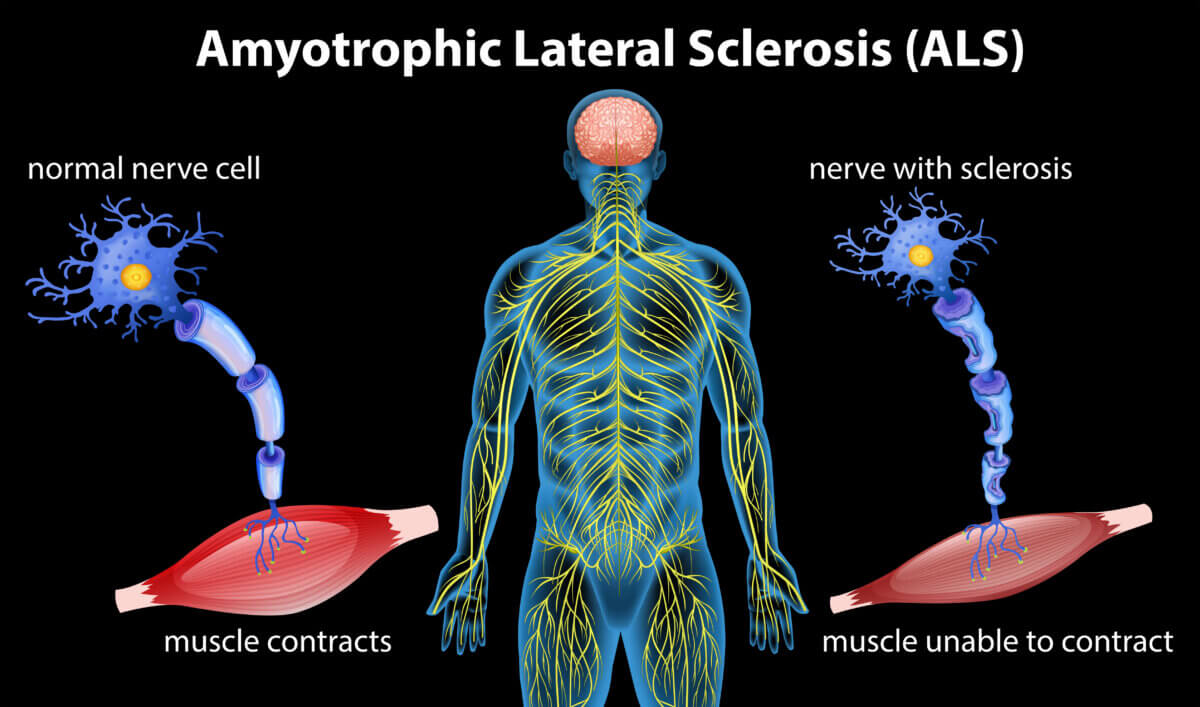
The team found that the mutation leads to two issues in the neuron. First, it makes the structures supporting the neuron’s axon (a slender cable that transmits electrical messages to other neurons) less stable, leaving it prone to collapse. Second, the mutation hampers the neuron’s ability to transport RNA or proteins, known as nuclear import, into its nucleus. Without the RNA – which relays DNA instructions – and vital proteins, the nucleus’s role in the cell’s function is compromised.
“By illuminating these two pathways, we’re suggesting these are great therapeutic targets for the disease,” says study lead author Evangelos Kiskinis, assistant professor of neurology and neuroscience at Northwestern University Feinberg School of Medicine, in a university release. “This discovery is important because a major breakthrough in ALS research in the last few years was discovering that nuclear import is disrupted in other forms of genetic ALS. We are linking this new cause of ALS to other genetic causes in which the same process is disrupted.”
An unaddressed question is whether ALS is one distinct disease or a grouping of genetically diverse subtypes.
“Our discovery — of the same destructive mechanisms in other genetic forms of ALS — leads us to believe this is the same disease,” says Kiskinis. “This new awareness is critical to developing treatments and for designing optimal clinical trials targeting specific ALS patient populations.”
ALS deteriorates the brain and spinal cord’s motor neurons, leading to paralysis and eventual death.
In an exciting turn, researchers tested anti-cancer drugs, known for stabilizing microtubules (the structural components of the nerve’s axon disrupted in ALS), on human ALS brain cells. While the drugs stabilized the microtubules and revived the nerve cells with the ALS mutation, using them directly to treat ALS may prove challenging due to potential severe side effects.
“This suggests that stabilizing microtubules is a rational therapeutic approach in ALS,” notes Kiskinis.
The team is now delving deeper into understanding NEK1’s role in ALS and exploring ways to enhance NEK1’s function to halt the disease’s progression.
The study is published in the journal Science Advances.
You might also be interested in:
- Discovery of 155 new genes reveals humans are still evolving!
- Cure For ALS On The Horizon After Promising Animal Study
- Virus remnants from 50 million years ago may be responsible for ALS

Research at the university of central Florida funded by WFND has documented that in human ALS neurons no matter what the gene mutation is lacks the electrophysiological signal in the axon to synapse or NMJ. Adding the Deanna protocol restores the signal increases cell energy.
Further every ALS patient whether familial or sporadic when properly treated is positive for a borrelia infection