OKINAWA, Japan — A cellular breakthrough may finally unlock the secrets of aging and regeneration. Scientists in Japan have discovered a previously unknown fate of damaged cells that could significantly change our understanding of aging, disease, and how our bodies heal themselves. This finding challenges the long-held belief that cells only have two possible outcomes when their membranes are damaged: either they recover — or they die.
The cell membrane is a delicate, flexible barrier only five nanometers thick (about 1/20 the thickness of a soap bubble). It acts like a gatekeeper, allowing certain substances to pass in and out while keeping others out. Though it protects the cell, it is vulnerable to damage from everyday activities such as muscle contractions and injuries. Researchers from the Okinawa Institute of Science and Technology (OIST) Graduate University explain that while cells have mechanisms to repair minor damage, not all damage leads to a straightforward path of recovery or death.
Instead, there’s a third outcome: cellular senescence, a state in which cells cease to divide but remain metabolically active, contributing to various body processes, both beneficial and harmful.
“When I started this project, I simply aimed to understand the repair mechanisms of damaged cell membrane,” recalls Professor Keiko Kono, head of the Membranology unit and senior author of this study, in a media release. “Unexpectedly, we ended up discovering that cell membrane damage, in a sense, switches cell fate.”
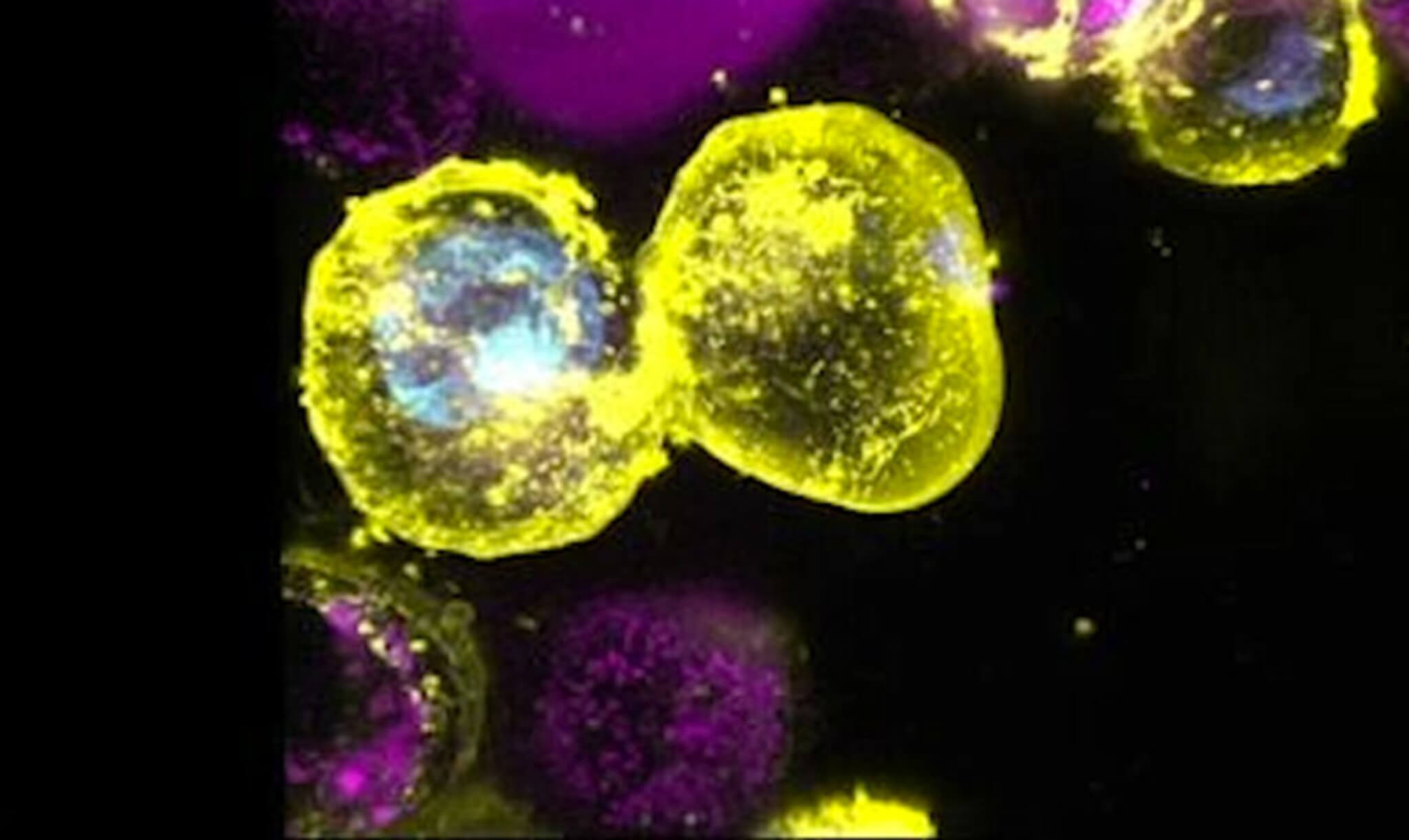
Testing Cell Membranes In Yeast
Using baker’s yeast as a model organism, the researchers identified 48 genes involved in the cell membrane repair process. They showed that yeast strains lacking some of these genes were more sensitive to a chemical that intentionally damages cell membranes. Intriguingly, these strains also had shortened replicative lifespans, meaning they underwent fewer rounds of cell division before growth arrest and death. Overexpression of certain membrane repair genes extended lifespan in normal yeast. This suggested a strong link between plasma membrane integrity and cellular aging.
The team then investigated whether plasma membrane damage influences aging in human cells using lung and skin fibroblasts grown in laboratory dishes. They utilized several methods to perforate the membranes, including detergents, bacterial toxins, and focused lasers. The fibroblasts responded by prematurely entering a state resembling natural cellular senescence, whereby they permanently withdraw from the cell cycle and adopt an aging phenotype. Senescent cells exhibit flattened morphology, increased beta-galactosidase enzyme activity, and secretion of inflammatory proteins called SASP factors. The researchers observed all these classic markers of senescence after membrane disruption.
When the cell membrane gets damaged, it allows calcium from outside the cell to rush inside. This calcium triggers the activation of p53, a key protein known as the “guardian of the genome” that regulates cell growth. p53 turns on production of another protein called p21 that acts like a brake pedal, forcing the cell cycle to a halt so the cell stops dividing. The scientists found they could prevent this premature aging response by using a compound that grabs onto calcium ions, preventing p53 activation. Interestingly, DNA damage usually activates p53 as well, but that pathway mediated by DNA repair processes didn’t seem essential here after membrane damage. So this reveals a novel route to p53 regulation.
The internal genetic programs of the senescent cells with leaky membranes also looked different compared to other types of senescent cells, like those that stopped dividing due to DNA mutations. One major difference was temporary increased activity of genes involved in wound healing. When the membrane-damage senescent cells were grown alongside young, dividing cells, they stimulated the migration and movement of the younger cells via secreted signaling factors. This makes the researchers speculate that in a body, cell membrane tears caused by injury could generate these membrane-damage senescent cells, which then facilitate faster closure and healing of the wound. This could explain why senescent cells often accumulate around wounds and damage sites—they may play a helpful role, at least initially.

Besides hallmarks of accelerated aging, the scientists observed another weird feature specific to the cell membrane and replicative senescent cells, which was tiny lipid-rich spots on the membrane surface. The spots come from a phospholipid called phosphatidylserine that flips from the inner to the outer membrane leaflet when cells get old or stressed. What these spots actually do is anyone’s guess at this point—but at the very least, they can serve as a distinct molecular marker to identify cells that became senescent due to membrane dysfunction versus other causes like DNA damage.
The research revealed that the fate of a cell post-damage depends on the extent of the damage and the resulting influx of calcium ions. Minor damage can be repaired, allowing cells to continue dividing. Severe damage leads to cell death. Intriguingly, moderate damage can initially look like it’ll be repaired, only for the cells to become senescent days later.
Power Of Cellular Senescence In Aging
Cellular senescence is a critical concept in understanding aging and disease. While cancer cells can divide without limit, normal cells can only divide about 50 times before they become senescent. These senescent cells don’t just sit idle, they produce proteins that can influence immune responses, impacting everything from wound healing to cancer and the aging process itself. The presence of senescent cells in the body and their role in health and disease have been areas of intense study, with research suggesting that removing these cells could rejuvenate bodily functions in experimental animals.
The traditional view scientists have held is that cellular senescence was triggered by repeated cell division or other stresses like DNA damage or other gene activations, typically involving a DNA damage response. However, this new study highlights a different pathway involving calcium ions and the tumor suppressor gene p53, brought on by damage to the cell membrane.
“The gene expression profile and bioinformatics suggested that cell membrane damage explains the origin of senescent cells in our bodies, specifically the ones near damaged tissues,” explains Prof. Kono.
Study authors believe their findings may contribute to the develop of a cellular treatment strategy which enhances human longevity in the future.
The findings are published in the journal Nature Aging.
