RIVERSIDE, Calif. — The new year is bringing new hope in the battle against cancer. Researchers at the University of California-Riverside have developed a novel method to control a notorious protein, MYC, which plays a pivotal role in the progression of three in four human cancer cases.
MYC, a protein without a defined shape, is crucial in normal cells for transcription — the process of converting genetic information from DNA to RNA and subsequently into proteins. However, in cancer cells, MYC’s activity becomes exaggerated and unregulated.
“Normally, MYC’s activity is strictly controlled. In cancer cells, it becomes hyperactive, and is not regulated properly,” explains study author Min Xue, an associate professor of chemistry at UC Riverside, in a university release. “MYC is less like food for cancer cells and more like a steroid that promotes cancer’s rapid growth. That is why MYC is a culprit in 75 percent of all human cancer cases.”
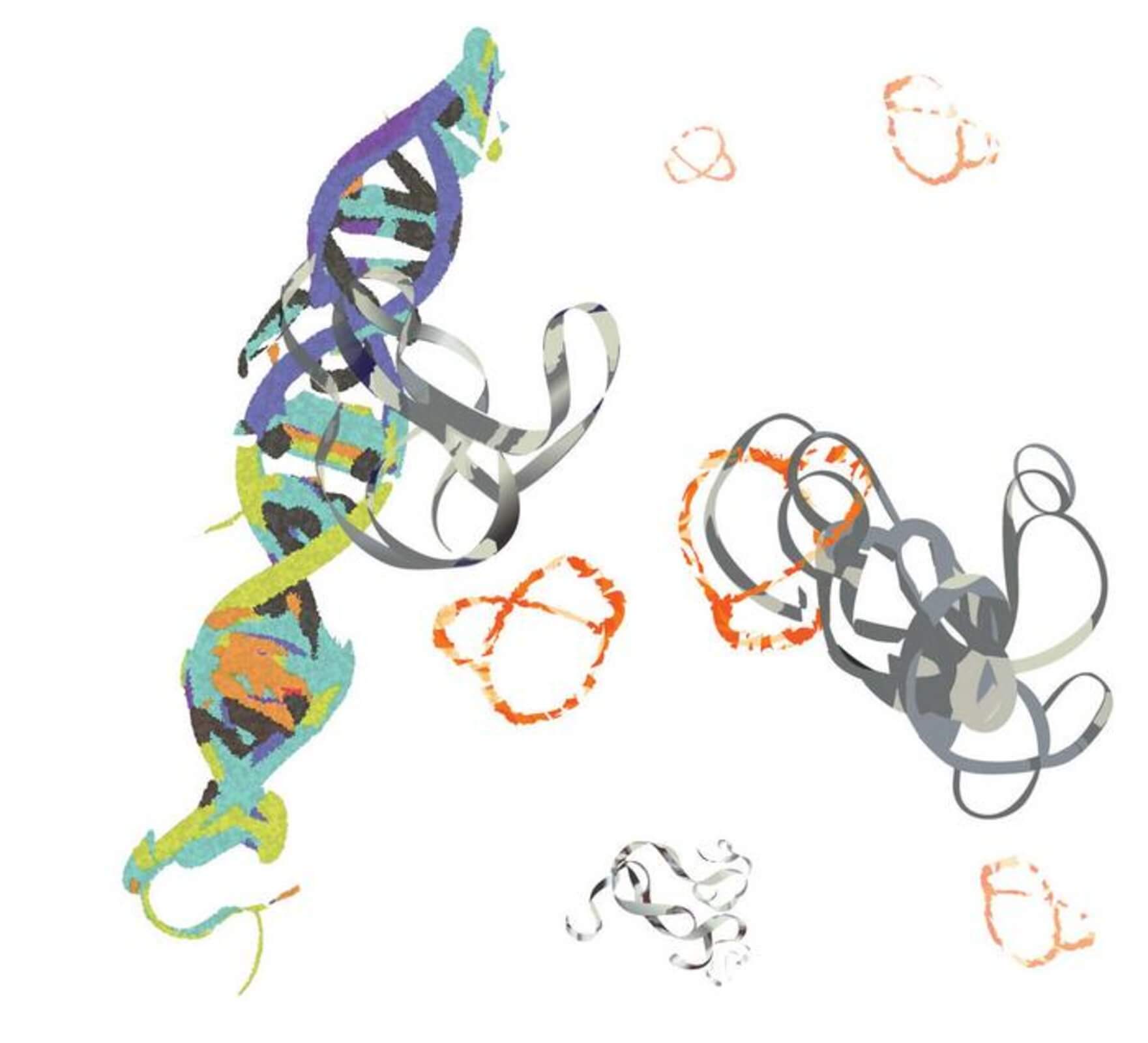
The challenge in targeting MYC lies in its structure — or lack thereof. Described by Xue as “a glob of randomness,” MYC’s shapeless form renders conventional drug development strategies ineffective. Traditional methods rely on well-defined structures to create drugs, but this approach falls short with MYC.
In a significant breakthrough, the UC Riverside team unveiled a peptide compound that successfully binds to MYC, suppressing its hyperactivity. This development is the result of years of research that began in 2018 when the team observed that altering a peptide’s rigidity and shape enhances its ability to interact with structureless proteins like MYC.
“Peptides can assume a variety of forms, shapes, and positions,” Xue explains. “Once you bend and connect them to form rings, they cannot adopt other possible forms, so they then have a low level of randomness. This helps with the binding.”
The new peptide demonstrates what is known as sub-micro-molar affinity — a highly specific and strong interaction nearing the strength of an antibody. The potency of this peptide marks a significant improvement over previous versions, bringing the team closer to their drug development goals.
“We improved the binding performance of this peptide over previous versions by two orders of magnitude,” Xue continues. “This makes it closer to our drug development goals.”
The current focus is on delivering the peptide into cells using lipid nanoparticles, which are tiny spheres made of fatty molecules. While not ideal for drug use, the team is actively working on enhancing the peptide’s ability to penetrate cells effectively. Once inside a cell, the peptide binds to MYC, altering its physical properties and inhibiting its role in transcription activities. This innovative approach promises a new era in cancer treatment, targeting the chaos that MYC represents.
“MYC represents chaos, basically, because it lacks structure. That, and its direct impact on so many types of cancer make it one of the holy grails of cancer drug development,” Xue concludes. “We are very excited that it is now within our grasp.”
The research was funded in part by the U.S. Department of Defense and the National Institutes of Health.
The study is published in the Journal of the American Chemical Society.
You might also be interested in:
- New cancer vaccine successfully boosts immune system, shrinks tumors in mice
- Revolutionary ‘nanodrones’ target and eliminate cancer cells
- One genetic mutation may be responsible for 20% of severe breast cancer cases

When cells lose energy they produce abnormal proteins, enzymes, and ultimately gene mutations. Why do cells lose energy? One cause is pathogens another is a lack of nutrients.
Research should focus on what can cause cells to lack energy not on the result which is abnormal products.